Introduction
Poultry primary processing in developed countries is commonly divided into the “dirty zone,” including stunning, bleeding, scalding, defeathering, and evisceration, and the “clean zone,” including washing and chilling (González-Miret et al., 2006). However, not all processing operations in developing countries can decontaminate and chill carcasses rapidly, which is required to control microbial loads (Belluco et al., 2016). Most European poultry processing plants use air-based chilling, whereas water immersion chilling is standard in the United States (Sánchez et al., 2002). Chilling is the final step in poultry primary processing because it is an essential stage to apply antimicrobials to reduce initial microbial count (Allen et al., 2000; Carroll and Alvarado, 2008). However, in developing countries such as Vietnam, most vendors in open markets (OM) and indoor markets (IM) harvest their birds and have neither the intention nor the resources to chill carcasses immediately (Burgos et al., 2007). On the contrary, vendors in the supermarkets (SM) receive frozen and packaged whole chickens from standardized and sanitary processing plants.
Poultry meat is the second most popular animal protein in Vietnam after pork. Approximately 1.5 thousand tons of poultry meat were produced in 2020 (General Statistics Office of Vietnam, 2021). The annual average poultry meat per capita consumption in Vietnam is 7.1 kg (General Statistics Office of Vietnam, 2021). When purchasing whole chickens in Vietnam, consumers prefer to keep the viscera and internal organs with the carcasses in the same bag. This poses serious microbiological safety implications because the gastrointestinal tracts of birds are colonized with bacterial pathogens such as Salmonella. Moreover, during slaughter, leakage of intestinal contents may occur, which leads to fecal contamination of meat (Adeyanju and Ishola, 2014). In the US, although various interventions are available and applied during poultry primary processing at critical control points to lower overall bacterial counts (Lues et al., 2007; Mead, 2004; Svobodová et al., 2012), Salmonella prevalence in young chicken meat has been observed at 3.8 % in 2013 and 2014. Moreover, Salmonella prevalence in ground chicken was consistently reported at 44.6%. Although whole chicken is one of the most commonly consumed poultry products in developing countries such as Vietnam, there has not been any comprehensive study on Listeria levels in whole chickens. A few researchers such as Kuan et al. (2013) reported a 20.8% to 33.3% incidence in chicken offal in Malaysia, which has a similar culinary culture of consuming offal to Vietnam. A few other authors have investigated bacterial pathogens in whole chickens in Vietnam (Luu et al., 2006; Ta et al., 2012; Van et al., 2007), but none has considered the impacts of market setting, time of purchase, and differences in merchandising of poultry meat. Therefore, the objective of the current study was to investigate the prevalence of Salmonella and Listeria and bacterial counts of generic Escherichia coli and coliforms at 2 sampling times and as influenced by vendors’ practices in 3 market types in Ho Chi Minh City (HCMC), Da Nang (DN), and Ha Noi (HN) in Vietnam.
Materials and Methods
Sample collection and preparation
Sample collection occurred between January and May of 2015 and followed a procedure similar to that described by McCain (2015) and McCain et al. (2015). Briefly, HCMC, DN, HN, and their surrounding areas were selected to represent regional variation in meat merchandising in Vietnam. The characteristics of SM, IM, and OM are described in Table 1. In addition to the differences in infrastructure, market types (SM, IM, OM) also differed in the marketing system, sources of products, methods of preservation, and display methods. Within each region, 2 of the most popular grocery markets per market type were selected, resulting in 6 markets per region. Domestically raised and processed whole chickens were purchased in each market at 2 sampling times, the opening time (T0) and 4 h after opening (T4). The opening time varied not only by region but also by individual markets, ranging from 4 and 5 AM (OM and IM) to 9 AM (SM). Five whole chickens averaging 1,000 g each were purchased individually from various vendors in each market at both sampling times, resulting in 180 samples. Vendors were randomized as described by McCain (2015) and McCain et al. (2015). Moreover, if a market had less than 5 vendors, at least one vendor was sampled repeatedly. There was no vendor randomization in SM because each SM was the sole poultry vendor; however, samples were purchased separately by different buyers. Moreover, whole chickens in SM were individually overwrapped on Styrofoam trays and displayed on refrigerated shelves. Vendors in IM and OM processed their birds at the time of purchase by defeathering, eviscerating, and rinsing carcasses in water. Samples were purchased individually and placed aseptically in sterile Nasco Poultry Rinse Bags (Nasco, Fort Atkinson, WI). Carcass surface temperature was recorded by a Fisher Scientific Traceable Infrared Thermometer Gun (Fisher Scientific, Waltham, MA). Bags were sealed, stored in an Igloo Super Tough Sportsman ice chest (Igloo, Katy, TX) with ice packs, and transported to a local university in each region. Weight of the whole chickens was recorded, and 90 mL of Buffered Peptone Water (BPW; 25.5 g/L; 3M, St. Paul, MN) was added to Nasco Poultry Rinse Bags (Nasco; Vipham et al., 2012). The volume of BPW used for the whole chicken rinse was evaluated previously by using a food color solution to ensure that it was sufficient to wash the surface and the body cavity of the whole chicken carcasses. Two sterile 15-mL polypropylene tubes (Greiner Bio-One, Monroe, NC) of BPW rinsate were transported on ice to HCMC University of Technology for further analysis.
Characteristics used to classify supermarkets (SM), indoor markets (IM), and open markets (OM) across 3 regions of Vietnam
| Market Type | |||
|---|---|---|---|
| Market Characteristics | SM | IM | OM |
| Multiple vendors | √ | √ | |
| Air-conditioning | √ | ||
| Refrigeration | √ | ||
| Walls | √ | √ | |
| Roof | √ | √ | |
| Clean water availability | √ | √ | √ |
√ = Existing characteristics.
Microbiological analysis
Salmonella was analyzed as described by McCain (2015) and McCain et al. (2015). Briefly, 2.5 mL of BPW rinsate was mixed with 22.5 mL of Salmonella Enrichment Broth (3M) in a sterile Whirl-Pak bag (Nasco) and incubated at 45°C for 24 h. After incubation, 1 mL of this solution was combined with 10 mL of Rappaport-Vassiliadis R10 Broth (RVR10; 3M) and incubated at 41.5°C for 24 h. A total of 10 μL of the incubated RVR10 solution was streaked onto 3M Petrifilm of the Salmonella Express System, and the Petrifilm was incubated at 41.5°C for 24 h. Presumptive positive Salmonella spp. colonies were identified as red colonies with yellow halo (3M, 2015c). Listeria spp. was detected as described by McCain et al. (2015). Similarly, a volume of 2.5 mL of BPW rinsate was combined with 22.5 mL of Demi-Fraser Listeria Enrichment Broth (3M) in a sterile Whirl-Pak bag (Nasco) and incubated at 30°C for 24 h. A volume of 0.1 mL of the incubated solution was spread onto an ALOA agar petri dish and incubated at 37°C for 24 h. Presumptive positive Listeria spp. colonies were identified as blue to green colonies with or without a halo. Analyses of aerobic bacteria (aerobic plate count [APC]), E. coli, and coliforms were performed as described by McCain et al. (2015). A volume of 15 μL of BPW rinsate was combined with 1,485 μL of sterile BPW broth and serially diluted (1:100). One milliliter (1 mL) of each dilution was spread onto an APC Petrifilm and E. coli/coliform Petrifilm. The Petrifilm was incubated at 35°C for 24 h. Colony-forming units (CFU) were counted according to 3M interpretation guides (3M, 2015a; 3M, 2015b).
Market characteristics
Outdoor temperature (°C), relative humidity (%), meat surface temperature (°C), type of retail display, availability of refrigeration, use of gloves and hairnets, knife cleaning, and water availability were recorded for individual samples on data collection forms.
Calculation and statistical analysis
Salmonella and Listeria prevalence was reported as the percentage of positive samples estimated by a logistic regression model. Counts of aerobic bacteria, E. coli, and coliforms were reported as log CFU/g of the carcass, as detailed by McCain (2015) and McCain et al. (2015). Market characteristic data were reported as a crude percentage without statistical analysis.
All statistical analyses were performed using a generalized linear mixed model estimated by the GLIMMIX procedure in SAS version 9.4 (SAS Institute, Inc., Cary, NC). Prevalence of Salmonella and Listeria were analyzed as a 3 × 2 factorial arrangement in a randomized complete block design with region as block, market type (SM, IM, and OM) and sampling time (T0 and T4) as 2 factors, and a specific market at a specific sampling time as experimental unit using logistic regression. However, in the same design, linear regression was used to analyze bacterial counts with whole chickens being the experimental unit. Market type, sampling time, and their interaction were the fixed effects, whereas region was the random effect. Means were separated by the protected t test by using the LSMEANS statement with the PDIFF option in the GLIMMIX procedure. Statistical significance was determined at P ≤ 0.10.
Results and Discussion
Aerobic, E. coli, and coliform counts
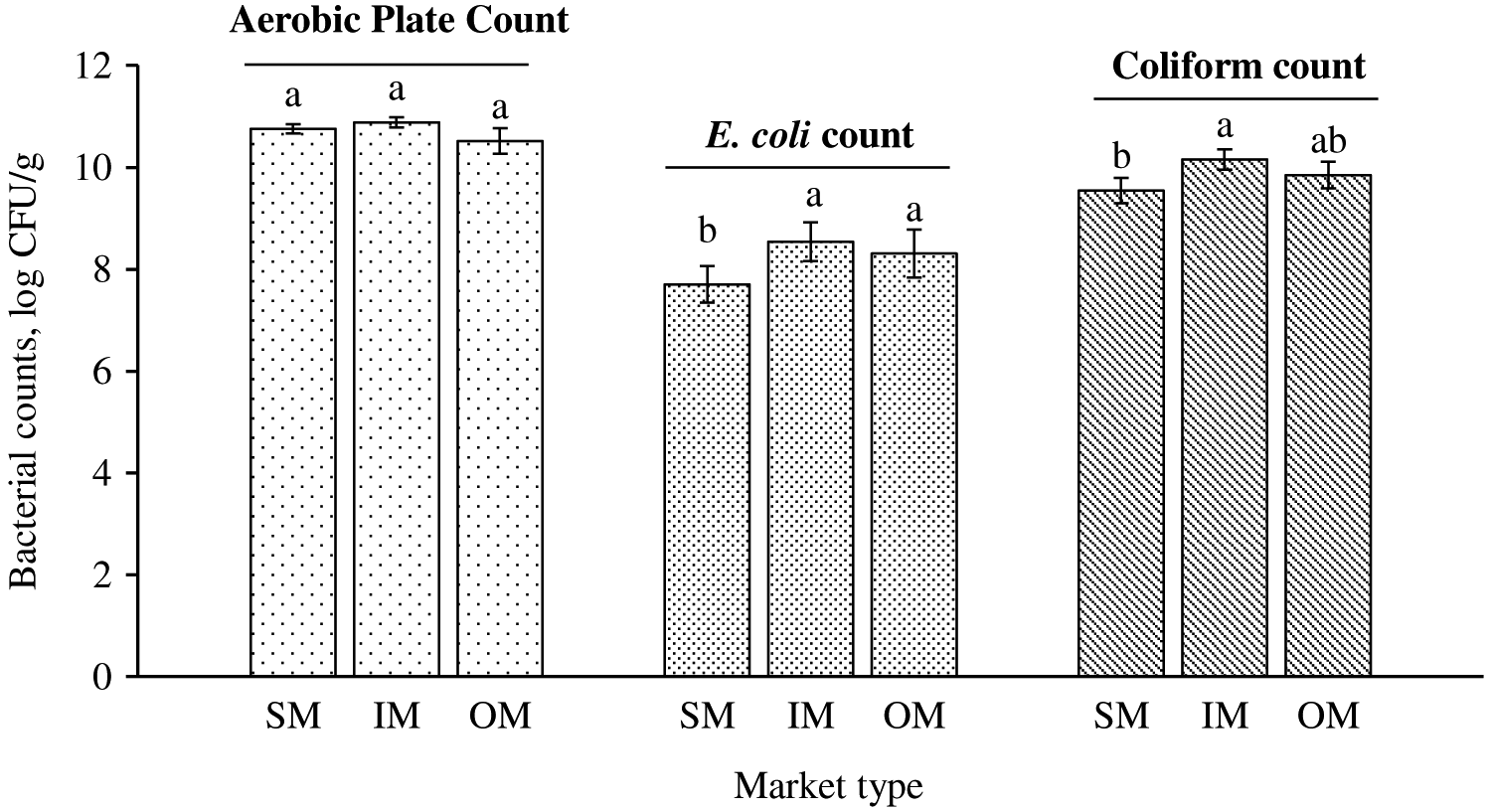
There was no difference in bacterial counts of APC, E. coli, or coliforms between 2 sampling times in all market types (P ≥ 0.170; Table 2). Some APC Petrifilm were too numerous to count at 10−6 dilution with pink color in the entire growth area (3M, 2015a) and therefore were estimated at 108 CFU, as recommended by the 3M guidelines. Average bacterial counts in whole chickens for all market types at T0 were 10.9, 8.1, and 9.9 log CFU/g of APC, E. coli, and coliforms, respectively (Table 2), and similar counts (P = 0.170) of 10.6, 8.3, and 9.8 log CFU/g at T4 were also observed. E. coli and coliform counts were greater in IM than in SM (P = 0.002 and 0.006, respectively; Figure 1). Furthermore, E. coli counts were also greater in OM than in SM (8.3 vs. 7.7 log CFU/g, respectively; P = 0.024), whereas both OM and SM had similar coliform counts (P = 0.170).
Aerobic plate count and E. coli and coliform counts of whole chickens (N = 180) purchased from supermarkets (SM), indoor markets (IM), and open markets (OM) in Ho Chi Minh City, Da Nang, and Ha Noi of Vietnam, averaged across 2 sampling times. Within a category of bacterial count, means without common letters differ (Pmarket type = 0.233, 0.006, 0.024, respectively). CFU = colony-forming units.
Bacterial counts and the prevalence of Salmonella and Listeria in whole chickens procured from supermarkets (SM), indoor markets (IM), and open markets (OM) at the market opening (T0) and 4 h after the opening (T4) across 3 regions of Vietnam (Ho Chi Minh City, Da Nang, and Ha Noi)
| SM | IM | OM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Microbiological Measurement* | T0 | T4 | T0 | T4 | T0 | T4 | Pmarket | Ptime | Pmarket × time |
| APC1, log CFU/g | 10.8 ± 0.1ax | 10.8 ± 0.1ax | 10.9 ± 0.0ax | 10.9 ± 0.0ax | 10.9 ± 0.0ax | 10.1 ± 0.5ax | 0.233 | 0.170 | 0.118 |
| E. coli2, log CFU/g | 7.5 ± 0.5ax | 7.9 ± 0.5ax | 8.4 ± 0.5bx | 8.7 ± 0.5bx | 8.3 ± 0.7bx | 8.3 ± 0.7bx | 0.006 | 0.281 | 0.619 |
| Coliform3, log CFU/g | 9.4 ± 0.4x | 9.7 ± 0.3ax | 10.1 ± 0.3bx | 10.2 ± 0.3bx | 10.1 ± 0.3abx | 9.6 ± 0.4abx | 0.024 | 0.874 | 0.162 |
| Salmonella4 prevalence, % | 25.6 ± 12.3ax | 35.8 ± 12.0ax | 57.0 ± 9.6bx | 60.5 ± 5.2bx | 35.8 ± 8.0ax | 35.8 ± 9.6ax | 0.013 | 0.515 | 0.822 |
| Listeria5 prevalence, % | 42.7 ± 16.7ax | 69.5 ± 13.3 ax | 73.1 ± 10.0bx | 83.1 ± 7.3bx | 73.1 ± 12.4bx | 73.1 ± 13.4bx | 0.060 | 0.113 | 0.415 |
Aerobic plate count, enumerated using 3M Petrifilm Aerobic Plate Count (3M).
Escherichia coli, enumerated using 3M Petrifilm E. coli/Coliform Count Plates (3M).
Coliform, enumerated using 3M Petrifilm E. coli/Coliform Count Plates (3M).
Salmonella, detected using 3M Petrifilm Salmonella Express System (3M).
Listeria, detected using ALOA media (bioMérieux, Hazelwood, MO)
Within market type, means without common letters differ, P ≤ 0.1.
Within sampling time, means without common letters differ, P ≤ 0.1.
Values were reported as estimated least squares means ± standard error of the means.
CFU = colony-forming units.
The high levels of bacterial counts indicated that the whole chickens in these markets had much higher initial bacterial loads than what is normally observed in developed countries. Good management practices during slaughtering and processing stages are important to minimize initial bacterial contamination (Buncic and Sofos, 2012). Understanding carcass hygiene or initial bacterial loads is very important to manage bacterial counts in poultry primary processing (Belluco et al., 2016). Aerobic bacteria and E. coli are commonly used as hygienic indicator organisms in poultry production (Adeyanju and Ishola, 2014). E. coli count was greater in Salmonella-positive beef and pork samples (Pearce et al., 2004; Ghafir et al., 2008; Biasino et al., 2018). Ghafir et al. (2008) suggested that E. coli count was a reliable index of Salmonella incidence in beef. In poultry carcasses, Salmonella prevalence has been routinely associated with processing hygiene, handling, and storage conditions (Whyte et al., 2004; Williams et al., 2015). The authors’ observations during the current study at IM and OM revealed that the conditions of cages used to house chickens before harvest and the water used to defeather birds and rinse carcasses could provide insights into increased levels of APC, E. coli, and coliforms. The cages and water were unsanitary with an abundance of fecal materials. Water used to rinse live chickens was also used for the final rinse of carcasses. In IM and OM, chilling was not available to all vendors, although some did have access to refrigeration (4°C) to store final products. Allen et al. (2000) observed a 1.28-log CFU/carcass reduction when water chilling was used. However, water chilling can also be a primary vehicle for foodborne pathogens (Demirok et al., 2013). Extensive bird-to-bird contact by water immersion chilling can result in pathogen cross-contamination to other carcasses (Bilgili et al., 2002). There is currently not much research in the US or other developed countries on E. coli enumeration in poultry, primarily because Salmonella, not E. coli, is a more challenging problem in the poultry industry. Moreover, there has not been any research on E. coli counts in whole chickens to be used as a hygienic indicator for market types in Vietnam. Therefore, the data in the current study provide important baseline information for the meat and poultry industries in Vietnam.
Prevalence of Salmonella
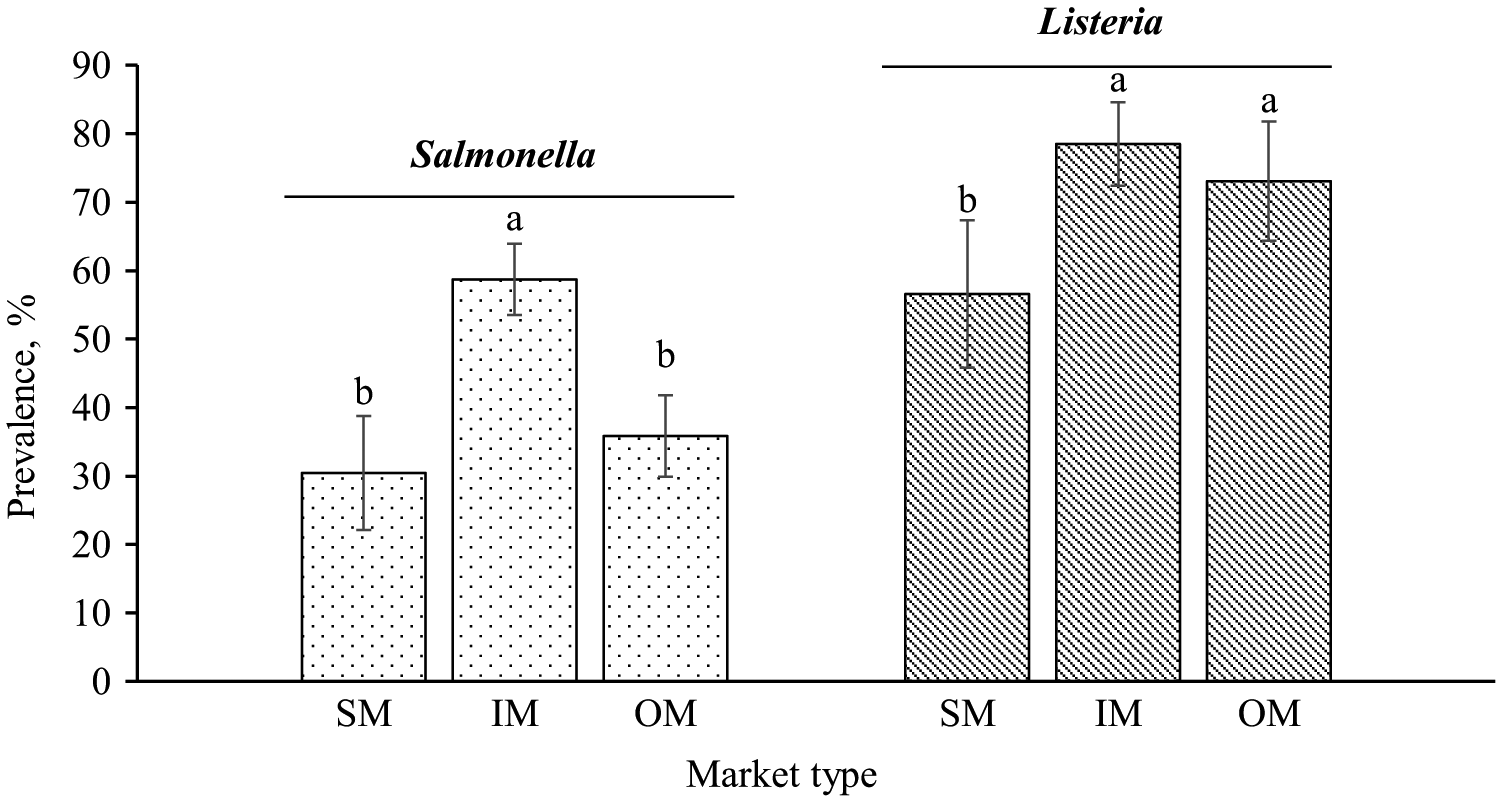
The prevalence of Salmonella ranged from 25.6% to 35.8%, 57.0% to 60.5%, and 35.8% in SM, IM, and OM, respectively (Table 2). Among market types, IM had 23% and 28% greater Salmonella prevalence than OM and SM, respectively (P = 0.006 and 0.022; Figure 2). Sampling time did not affect Salmonella prevalence (P = 0.515), and there was no interactive effect of market type and sampling time (P = 0.822).
Salmonella and Listeria prevalence in whole chickens (N = 180) purchased from supermarkets (SM), indoor markets (IM), and open markets (OM) in Ho Chi Minh City, Da Nang, and Ha Noi of Vietnam, averaged across 2 sampling times. Within a pathogen category, means without common letters differ (Pmarket type = 0.013 and 0.060, respectively).
Currently, Vietnam does not have a foodborne disease surveillance system to monitor the annual incidence of human salmonellosis (Ta et al., 2012). Salmonella is a major cause of foodborne disease worldwide, especially in Southeast Asia (CDC, 2015; Ta et al., 2012). Salmonella is isolated more from raw poultry than from other foods (CDC, 2015) because the bacteria naturally occur in the intestinal tract of birds. A few studies have been published in Vietnam to investigate Salmonella in chicken; however, they are limited in geographical variation and sample size (Phan et al., 2005; Luu et al., 2006; Van et al., 2007; Ta et al., 2012). These authors reported 48.9%, 53.3%, 21.0%, and 45.9% prevalence, respectively, which were comparable to the levels found in whole chickens in the current study. However, the data collected throughout the country from 2005 to 2012 indicated a certain degree of variation. Van et al. (2007) reported a 21.0% prevalence compared with 53.3 % reported by Luu et al. (2006). Ta et al. (2012) reported 51.1% (N = 239), 45.5% (N = 33), and 44.7% (N = 264) prevalence in HN, DN, and HCMC, respectively. Averaged across all markets and sampling times for each region, the current study showed that Salmonella prevalence in HN, DN, and HCMC was at 47.1%, 25.0%, and 55.0%, respectively, similar to findings by Ta et al. (2012). Market types in Vietnam vary primarily in the processing and handling of poultry carcasses. SM receive frozen retail products that may have undergone interventions for decontamination and chilling. Vendors in IM and OM markets do not have the infrastructure or financial resources to decontaminate and chill whole chicken carcasses. This might explain the greater bacterial loads and pathogenic prevalence in the IM and OM compared to the SM. Moreover, a study conducted in wet markets in China reported 52.2% Salmonella prevalence, approximately 16.4% greater than the results in the current study (Yang et al., 2011). Retail surveys have revealed that Salmonella prevalence on raw chicken carcasses and retail meats, although varied among countries, tends to be higher in market settings without cold storage. In Ethiopia, chicken meats and giblets had a 68.2% positive rate (Tibaijuka et al., 2003), although chicken meat samples were only positive at a 28% rate. Barbour et al. (2015) reported 53.3% prevalence in chicken carcasses in 6 developing countries, with a 43.8% positive rate in SM and 50.0% from street vendors. In Portugal, Antunes et al. (2003) reported 60% Salmonella prevalence in chicken carcasses collected at 3 local sources, however, representing whole chickens from several national processors. These authors, instead of rinsing the whole carcasses, only sampled portions of skin from the neck and cloacal regions. The samples in these studies were from local sources without refrigeration and/or microbial control postharvest. Domínguez et al. (2002) also sampled the skin of chicken carcasses from retail outlets in Spain, which are mostly refrigerated (Álvarez-Astorga et al., 2002) at the time of sampling, and reported 35.8% prevalence. These authors acknowledged that the variation in Salmonella prevalence might be caused by sampling methods and culture techniques. However, in the greater Washington, DC, area in the US, Salmonella prevalence in whole chicken carcasses was only 4.2% (Zhao et al., 2001). These authors confirmed presumptive Salmonella colonies by polymerase chain reaction. In 2016, the US Food Safety and Inspection Service proposed a 15.4% positive rate as the performance standard for Salmonella in chicken parts (Federal Register, 2016). The most recent posting indicated that 64% of all processing plants met or exceeded this performance standard (FSIS, 2021), although there might be a great variation in Salmonella prevalence from 2.5% to 60% in postchill carcasses (Berrang et al., 2009). Most US processing plants employ various antimicrobials and carcass chilling to reduce Salmonella and Campylobacter prevalence (Kim et al., 2017).
Water washing is also important in poultry processing. This step can either decrease or increase the bacterial load. Increased bacterial loads, especially those of E. coli, could lead to increased Salmonella prevalence (Ghafir et al., 2008). A Spearman’s rank correlation conducted in the current study revealed a correlation between Salmonella prevalence and E. coli counts (r = 0.52; P = 0.001). Moreover, proper washing with clean water has been shown to decrease Salmonella prevalence by the physical removal of injured or semi-attached cells (Cox et al., 2010). However, in IM and OM, clean water was not always readily available. Carcasses were rinsed in the same water throughout the day. This practice can lead to cross-contamination among poultry carcasses (Kuan et al., 2013). Yang et al. (2011) observed that wet markets in China, similar to OM in Vietnam, also had a limited supply of potable water. These authors reported that the eviscerated birds were rinsed with a minimal amount of water or dipped in a tank without frequent change of water. The vendors at these Chinese wet markets were so busy that they rarely had time to wash their hands, scales, and other tools. Therefore, it was suggested that cross-contamination between chicken carcasses with Salmonella was likely to cause an increase in Salmonella prevalence (Yang et al., 2011). This same observation could also be attributed to the prevalence of Salmonella in the current study, in which only 26.7% and 6.7% of IM and OM vendors, respectively, wore gloves. Gloves in these markets were used to keep the vendors’ hands clean rather than to maintain carcass hygiene. Many consumers are not aware of the safety risks associated with contamination of raw chicken with Salmonella because chicken is usually cooked thoroughly by boiling before consumption (Othman, 2007). However, chicken meat is usually associated with direct hand-to-mouth exposure to pathogens and cross-contamination in the food preparation area (Yang et al., 2011). In the US, an estimated 11% of human Salmonella infections annually are attributed to exposure to live poultry (USDA, 2014), and 17% of all foodborne illnesses are associated with poultry (Painter et al., 2013). Moreover, undercooked chicken is a major source of salmonellosis (Yang et al., 2016). The potential risks of foodborne illnesses to consumers at retail markets could be decreased by implementing good manufacturing processing practices throughout the poultry production chain.
Prevalence of Listeria
Listeria prevalence in each market type at a specific sampling time is reported in Table 2. Listeria prevalence in the whole chickens ranged from 42.7% to 69.5%, 73.1% to 83.1%, and 73.1% in SM, IM, and OM, respectively. The prevalence of Listeria was lower in SM than in IM and OM (P = 0.024 and 0.089, respectively; Figure 2). There was no effect of sampling time or market type × sampling time interaction on Listeria prevalence (P = 0.113 and 0.415, Table 2).
There was a great within-market variation in Listeria contamination. The high level of Listeria prevalence might be attributed to the widespread occurrence of Listeria spp. in the environment (Chiarini et al., 2009) because Listeria survives harsh conditions in food processing plants for an extended time (Loura et al., 2005; Lundén et al., 2003). Moreover, L. monocytogenes can colonize the floor drains and persist in this environment for years (Berrang et al., 2013). Recent research on the prevalence of Listeria in whole chickens is not widely available in both developed and developing countries. Salmonella is studied more because it is present in the intestines of birds, whereas Listeria is mostly from environmental contamination. Listeria prevalence in raw broiler carcasses was reported to be from 41% to 84% (Uyttendaele et al., 1999). Studies have indicated that improper cleaning and disinfecting of equipment in poultry processing facilities can lead to the contamination of poultry carcasses (Adeyanju and Ishola, 2014; Loura et al., 2005). Uytttendaele et al. (1999) reported an increase in Listeria contamination rate as carcasses moved through cutting and further processing. Contamination rates of whole carcasses, carcasses with parts, and retail products were 41.3%, 46.7%, and 61.0%, respectively (Uytttendaele et al., 1999). Furthermore, additional handling of poultry carcasses during processing, especially after chilling, has been shown to be responsible for an increase in prevalence at the end of the processing line (Genigeorgis et al., 1990). This might help explain the high level of 42.7% to 69.6% prevalence of Listeria in SM, even though SM vendors received prepackaged frozen or refrigerated whole chickens, which remained refrigerated throughout the retail display. Contamination at the end of the processing line and the psychrotrophic nature of Listeria (Chiarini et al., 2009) explain the high prevalence of poultry carcasses in SM. However, it was still less than in IM and OM because poultry products in these 2 market types were temperature-abused. Listeria has been isolated from raw poultry (Miettinen et al., 2001); however, prevalence is highly varied. Pini and Gilbert (1988) found 60% of Listeria-contaminated raw chickens in the United Kingdom, whereas Bailey et al. (1989) only observed 23% prevalence in raw poultry carcasses in retail establishments in the US. Moreover, Loncarevic et al. (1994) reported 0% to 64% prevalence of Listeria in raw broiler meat. The results from the current study were slightly greater than what was found in the previously mentioned studies. The current study was conducted in the retail setting, whereas others were in processing facilities. Miettinen et al. (2001) observed Listeria contamination in processing facilities at as low as 1% to 11% but as high as 62% in retail establishments. The increased prevalence at retail could be attributed to either poor vendor hygiene or the duration of retail display. Genigeorgis et al. (1989) observed workers’ hygiene in poultry processing facilities and reported that 46.7% of the workers harbored Listeria spp. in their hands and gloves. Moreover, Loura et al. (2005) sampled the bare hands of food handlers and reported that 60% of samples had Listeria. Hygienic conditions in all markets in Vietnam were poor with only 16.7%, 16.7%, and 6.7% of SM, IM, and OM vendors at T0 and 16.7%, 26.7%, and 3.3% of SM, IM, and OM vendors at T4 using gloves, respectively. Hygienic conditions of IM and OM vendors might be the reason for the 15% and 20% greater Listeria prevalence in OM and IM, respectively, than in SM. It is recommended to cook poultry products to 74°C (FSIS, 2014), thereby assuming a low risk of Listeria.
Market characteristics
Characteristics of markets and vendors in Vietnam are summarized in Table 3. Cover displays for whole chickens were overwrapped packages or display cases as a physical barrier between the products and the consumers; both were only used at some SM and IM vendors. At T0, 50.0% and 73.3% of the SM and IM vendors used cover displays, respectively. However, at T4, 83.3% and 20.0% SM and IM used cover displays. Moreover, 100.0% of OM vendors exposed whole chickens to open air at both sampling times. From observations during sampling, if the chickens were harvested at the market, whole chickens were hung by feet after processing and displayed openly without wrapping until closing. If not being sold by the end of the day, the carcasses were overwrapped and stored for sale the next day. Likewise, in SM, whole chickens were processed at a central location, overwrapped, and frozen for transportation to SM. Refrigeration was used by 50.0%, 66.7%, and 6.7% of SM, IM, and OM vendors at T0 and by 100.0%, 23.3%, and 0.0% of SM, IM, and OM vendors at T4, respectively. Refrigeration was used in SM for display at T0 but for storage at T4. However, refrigeration in IM and OM was used for storage only. As mentioned previously, hygienic conditions in all markets were poor with only 16.7%, 16.7%, and 6.7% of SM, IM, and OM vendors at T0 and 16.7%, 26.7%, and 3.3% of SM, IM, and OM vendors at T4 using gloves. Chicken carcasses were rarely further processed because they were sold as whole birds. Furthermore, only 20.0% of OM vendors at T0 and 10% and 30% of OM and IM vendors at T4 cleaned knives. Because whole chickens were shipped to SM frozen and packaged, there was no knife usage in SM (0.0%). Hot water was used to wash knives by SM and IM only at T0 at 3.3% and 16.7%, respectively, whereas 10.0% and 23.3% of OM vendors used hot water at T0 and T4, respectively. Freshwater was available and used by 21.7%, 25.0%, and 10.0% of SM, IM, and OM vendors at T0 and 41.7%, 20.0%, and 5.0% at T4 by SM, IM, and OM vendors, respectively.
Observational and environmental data collected during the purchase of whole chickens from supermarkets (SM), indoor markets (IM), and open markets (OM) at the market opening (T0) and 4 h after the opening (T4) across 3 regions of Vietnam (Ho Chi Minh City, Da Nang, and Ha Noi)
| SM | IM | OM | ||||
|---|---|---|---|---|---|---|
| Market Characteristics | T0 | T4 | T0 | T4 | T0 | T4 |
| Outdoor temperature, °C | 27.8 ± 1.0 | 29.0 ± 1.7 | 25.2 ± 0.5 | 28.7 ± 0.7 | 27.4 ± 1.7 | 31.2 ± 1.6 |
| Humidity, % | 68.2 ± 5.6 | 59.3 ± 3.8 | 83.3 ± 4.6 | 70.5 ± 5.7 | 73.3 ± 5.7 | 63.1 ± 5.7 |
| Meat surface temperature, °C | 15.4 ± 1.6 | 14.5 ± 2.6 | 24.2 ± 1.8 | 21.8 ± 2.0 | 30.7 ± 5.5 | 25.9 ± 1.3 |
| Covered display, % | 50.0 ± 0.2 | 83.3 ± 0.1 | 73.3 ± 0.2 | 20.0 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Hang display, % | 0.0 ± 0.0 | 13.3 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Open display, % | 50.0 ± 0.2 | 30.0 ± 0.2 | 26.7 ± 0.2 | 80.0 ± 0.2 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| Refrigeration, % | 50.0 ± 0.2 | 100.0 ± 0.0 | 66.7 ± 0.2 | 23.3 ± 0.2 | 6.7 ± 0.1 | 0.0 ± 0.0 |
| Gloves, % | 16.7 ± 0.2 | 16.7 ± 0.2 | 16.7 ± 0.2 | 26.7 ± 0.2 | 6.7 ± 0.1 | 3.3 ± 0.0 |
| Hairnet, % | 33.3 ± 0.2 | 66.7 ± 0.2 | 50.0 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Cleaned knife before cutting, % | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 30.0 ± 0.2 | 20.0 ± 0.2 | 10.0 ± 0.0 |
| Hot water, % | 3.3 ± 0.0 | 0.0 ± 0.0 | 16.7 ± 0.2 | 0.0 ± 0.0 | 10.0 ± 0.1 | 23.3 ± 0.1 |
| Fresh water, % | 21.7 ± 0.1 | 41.7 ± 0.1 | 25.0 ± 0.1 | 20.0 ± 0.1 | 10.0 ± 0.1 | 5.0 ± 0.0 |
Vendors in IM and OM provide reasonably priced and conveniently available poultry products for the lower-income population. Chamhuri and Batt (2013) reported that consumers in Malaysia still preferred to shop at open and street vendors even though they were informed that meat in those markets was not as safe as meat in SM (Chamhuri and Batt, 2013). This attitude is important for the policymakers in Vietnam to consider because whole chicken is the most popular poultry product in Vietnam and much of the poultry supply chain was processed in poor hygienic conditions. Cross-contamination to other foods and risks of salmonellosis and listeriosis will increase tremendously if the whole chicken is not cooked properly.
Conclusion
Whole chickens had high levels of bacteria and a high prevalence of Salmonella and Listeria. The prevalence of Salmonella and Listeria in whole chickens in various market types in Vietnam was greater than data from the current literature. Furthermore, there were 7.5 to 10.9 log CFU/g of indicator organisms such as aerobic bacteria, E. coli, and coliforms, among which E. coli counts were correlated with Salmonella prevalence. Refrigeration, cleanliness, water usage, and good hygienic conditions can improve the status of microbiological safety and quality of poultry products in Vietnam. Much research is needed to establish a baseline for contamination at critical control points during poultry processing in Vietnam’s meat markets. These data justify enhanced enforcement of food safety regulations and the creation of educational programs for both consumers and vendors in Vietnam’s meat markets.
Acknowledgements
The current study was funded in part by the US Borlaug Fellows in Global Food Security Program Graduate Research Grant (Grant #00000861). Work in Dr. Janet R. Donaldson’s laboratory was supported by National Institutes of Health #P20GM103646. Microbiological training was provided by the International Center for Food Industry Excellence at Texas Tech University.
Literature Cited
3M. 2015a. 3MTM PetrifilmTM Aerobic Count Plate. https://www.3m.com/3M/en_US/p/d/b00013927/. (Accessed 15 December 2021.)
3M. 2015b. 3MTM PetrifilmTM E. coli/Coliform Count Plate. https://www.3m.com/3M/en_US/p/d/b00013933/. (Accessed 15 December 2021.)
3M. 2015c. 3MTM PetrifilmTM Salmonella Express System. http://solutions.3m.com/wps/portal/3M/en_US/Microbiology/FoodSafety/promotions/petrifilm-Salmonella-express/. (Accessed 15 December 2021.)
Adeyanju, G. T., and O. Ishola. 2014. Salmonella and Escherichia coli contamination of poultry meat from a processing plant and retail markets in Ibadan, Oyo State, Nigeria. Springerplus. 3:139. doi: https://doi.org/10.1186/2193-1801-3-139.
Allen, V. M., J. E. L. Corry, C. H. Burton, R. T. Whyte, and G. C. Mead. 2000. Hygiene aspects of modern poultry chilling. Int. J. Food Microbiol. 58:39–48. doi: https://doi.org/10.1016/s0168-1605(00)00259-2.
Álvarez-Astorga, M., R. Capita, C. Alonso-Calleja, B. Moreno, M. Del, and C. García-Fernández. 2002. Microbiological quality of retail chicken by-products in Spain. Meat Sci. 62:45–50. doi: https://doi.org/10.1016/S0309-1740(01)00225-X.
Antunes, P., C. Réu, J. C. Sousa, L. Peixe, and N. Pestana. 2003. Incidence of Salmonella from poultry products and their susceptibility to antimicrobial agents. Int. J. Food Microbiol. 82:97–103. doi: https://doi.org/10.1016/s0168-1605(02)00251-9.
Bailey, J. S., D. L. Fletcher, and N. A. Cox. 1989. Recovery and serotype distribution of Listeria monocytogenes from broiler chickens in the southeastern United States. J. Food Protect. 52:148–150. doi: https://doi.org/10.4315/0362-028X-52.3.148.
Barbour, E. K., D. B. Ayyash, W. Alturkistni, A. Alyahiby, S. Yaghmoor, A. Iyer, J. Yousef, T. Kumosani, and S. Harakeh. 2015. Impact of sporadic reporting of poultry Salmonella serovars from selected developing countries. J. Infect. Dev. Countr. 9:1–7. doi: https://doi.org/10.3855/jidc.5065.
Belluco, S., L. Barco, A. Roccato, and A. Ricci. 2016. Escherichia coli and Enterobacteriaceae counts on poultry carcasses along the slaughterline: A systematic review and meta-analysis. Food Control. 60:269–280. doi: https://doi.org/10.1016/j.foodcont.2015.07.033.
Berrang, M. E., J. S. Bailey, S. F. Altekruse, W. K. Shaw, Jr., B. L. Patel, R. J. Meinersmann, and P. J. Fedorka-Cray. 2009. Prevalence, serotype, and antimicrobial resistance of Salmonella on broiler carcasses postpick and postchill in 20 U.S. processing plants. J. Food Protect. 72:1610–1615. doi: https://doi.org/10.4315/0362-028x-72.8.1610.
Berrang, M. E., J. F. Frank, and R. J. Meinersmann. 2013. Contamination of raw poultry meat by airborne Listeria originating from a floor drain. J. Appl. Poultry Res. 22:132–136. doi: https://doi.org/10.3382/japr.2012-00676.
Biasino, W., L. De Zutter, W. Mattheus, S. Bertrand, M. Uyttendaele, and I. Van Damme. 2018. Correlation between slaughter practices and the distribution of Salmonella and hygiene indicator bacteria on pig carcasses during slaughter. Food Microbiol. 70:192–199. doi: https://doi.org/10.1016/j.fm.2017.10.003.
Bilgili, S. F., A. L. Waldroup, D. Zelenka, and J. E. Marion. 2002. Visible ingesta on prechill carcasses does not affect the microbiological quality of broiler carcasses after immersion chilling. J. Appl. Poultry Res. 11:233–238. doi: https://doi.org/10.1093/japr/11.3.233.
Buncic, S., and J. Sofos. 2012. Interventions to control Salmonella contamination during poultry, cattle and pig slaughter. Food Res. Int. 45:641–655. doi: https://doi.org/10.1016/j.foodres.2011.10.018.
Burgos, S., P. H. Hanh, D. Roland-Holst, and S. A. Burgos. 2007. Characterization of poultry production systems in Vietnam. International Journal of Poultry Science. 6:709–712. doi: https://doi.org/10.3923/ijps.2007.709.712.
Carroll, C. D., and C. Z. Alvarado. 2008. Comparison of air and immersion chilling on meat quality and shelf life of marinated broiler breast fillets. Poultry Sci. 87:368–372. doi: https://doi.org/10.3382/ps.2007-00213.
Centers for Disease Control and Prevention (CDC). 2015. National enteric disease surveillance: Salmonella annual report, 2015. https://www.cdc.gov/nationalsurveillance/pdfs/2015_SalmonellaREPORT-508.pdf. (Accessed 15 December 2016.)
Chamhuri, N., and P. J. Batt. 2013. Exploring the factors influencing consumers’ choice of retail store when purchasing fresh meat in Malaysia. Int. Food Agribus. Man. 16:99–122.
Chiarini, E., K. Tyler, J. M. Farber, F. Pagotto, and M. T. Destro. 2009. Listeria monocytogenes in two different poultry facilities: Manual and automatic evisceration. Poultry Sci. 88:791–797. doi: https://doi.org/10.3382/ps.2008-00396.
Cox, N. A., L. J. Richardson, J. A. Cason, R. J. Buhr, Y. Vizzier-Thaxton, D. P. Smith, P. J. Fedorka-Cray, C. P. Romanenghi, L. V. B. Pereira, and M. P. Doyle. 2010. Comparison of neck skin excision and whole carcass rinse sampling methods for microbiological evaluation of broiler carcasses before and after immersion chilling. J. Food Protect. 73:976–980. doi: https://doi.org/10.4315/0362-028x-73.5.976.
Demirok, E., G. Veluz, W. V Stuyvenberg, M. P. Castañeda, A. Byrd, and C. Z. Alvarado. 2013. Quality and safety of broiler meat in various chilling systems. Poultry Sci. 92:1117–1126. doi: https://doi.org/10.3382/ps.2012-02493.
Domínguez, C., I. Gómez, and J. Zumalacárregui. 2002. Prevalence of Salmonella and Campylobacter in retail chicken meat in Spain. Int. J. Food Microbiol. 72:165–168. doi: https://doi.org/10.1016/S0168-1605(01)00638-9.
Federal Register. 2016. New performance standards for Salmonella and Campylobacter in not-ready-to-eat comminuted chicken and turkey products and raw chicken parts and changes to related agency verification procedures: Response to comments and announcement of implementation schedule. https://www.federalregister.gov/documents/2016/02/11/2016-02586/new-performance-standards-for-salmonella-and-campylobacter-in-not-ready-to-eat-comminuted-chicken. (Accessed 25 September 2021.)
FSIS. 2014. Controlling Listeria monocytogenes in post-lethality exposed ready-to-eat meat and poultry products. FSIS-GD-2014-0001. Food Safety and Inspection Service.
FSIS. 2021. Salmonella verification testing: August 30, 2020 through August 28, 2021. https://www.fsis.usda.gov/news-events/publications/salmonella-verification-testing-august-30-2020-through-august-28-2021. (Accessed 25 September 2021.)
General Statistics Office of Vietnam. 2021. Sản lượng sản phẩm chăn nuôi chủ yếu. https://www.gso.gov.vn/px-web-2/?pxid=V0640&theme=N%C3%B4ng%2C%20l%C3%A2m%20nghi%E1%BB%87p%20v%C3%A0%20th%E1%BB%A7y%20s%E1%BA%A3n. (Accessed 25 September 2021.)
Genigeorgis, C. A., P. Oanca, and D. Dutulescu. 1990. Prevalence of Listeria spp. in turkey meat at the supermarket and slaughterhouse level. J. Food Protect. 53:282–288. doi: https://doi.org/10.4315/0362-028X-53.4.282.
Ghafir, Y., B. China, K. Dierick, L. De Zutter, and G. Daube. 2008. Hygiene indicator microorganisms for selected pathogens on beef, pork, and poultry meats in Belgium. J. Food Protect. 71:35–45. doi: https://doi.org/10.4315/0362-028x-71.1.35.
González-Miret, M. L., M. L. Escudero-Gilete, and F. J. Heredia. 2006. The establishment of critical control points at the washing and air chilling stages in poultry meat production using multivariate statistics. Food Control. 17:935–941. doi: https://doi.org/10.1016/j.foodcont.2005.06.012.
Kim, S. A., S. H. Park, S. I. Lee, C. M. Owens, and S. C. Ricke. 2017. Assessment of chicken carcass microbiome responses during processing in the presence of commercial antimicrobials using a next generation sequencing approach. Sci. Rep.-UK. 7:43354. doi: https://doi.org/10.1038/srep43354.
Kuan, C. H., S. G. Goh, Y. Y. Loo, W. S. Chang, Y. L. Lye, S. Puspanadan, J. Y. H. Tang, Y. Nakaguchi, M. Nishibuchi, N. A. Mahyudin, and S. Radu. 2013. Prevalence and quantification of Listeria monocytogenes in chicken offal at the retail level in Malaysia. Poultry Sci. 92:1664–1669. doi: https://doi.org/10.3382/ps.2012-02974.
Loncarevic, S., W. Tham, and M.-L. Danielsson-Tham. 1994. Occurrence of Listeria species in broilers pre- and post-chilling in chlorinated water at two slaughterhouses. Acta Vet. Scand. 35:149–154. doi: https://doi.org/10.1186/BF03548342.
Loura, C. A. C., R. C. C. Almeida, and P. F. Almeida. 2005. The incidence and level of Listeria spp. and Listeria monocytogenes contamination in processed poultry at a poultry processing plant. J. Food Safety 25:19–29. doi: https://doi.org/10.1111/j.0149-6085.2005.25551.x.
Lues, J. F. R., M. M. Theron, P. Venter, and M. H. R. Rasephei. 2007. Microbial composition in bioaerosols of a high-throughput chicken-slaughtering facility. Poultry Sci. 86:142–149. doi: https://doi.org/10.1093/ps/86.1.142.
Lundén, J. M., T. J. Autio, A.-M. Sjöberg, and H. J. Korkeala. 2003. Persistent and nonpersistent Listeria monocytogenes contamination in meat and poultry processing plants. J. Food Protect. 66:2062–2069. doi: https://doi.org/10.4315/0362-028X-66.11.2062.
Luu, Q. H., F. Reinhard, P. Padungtod, H. T. Tran, M. N. Kyule, M. P. O. Baumann, and K. H. Zessin. 2006. Prevalence of Salmonella in retail chicken meat in Hanoi, Vietnam. Ann. N. Y. Acad. Sci. 81(1): 257–261.
McCain, A. K. 2015. Influence of market setting and time of purchase on counts of aerobic bacteria, Escherichia coli, and coliform and prevalence of Salmonella and Listeria in beef, pork, and chicken in Vietnam. M.S. thesis, Mississippi State Univ., Mississippi State, MS.
McCain, A. K., P. T. T. Vu, T. T. M. Tran, M. V. V Le, D. H. Nguyen, P. R. Broadway, L. M. Guillen, M. M. Brashears, J. R. Donaldson, M. W. Schilling, and T. T. N. Dinh. 2015. Influence of market setting and time of purchase on bacterial counts and prevalence of Salmonella and Listeria in pork in Vietnam. Agric. Food Anal. Bacteriol. 5:166–182.
Mead, G. C. 2004. Microbial hazards in production and processing. In: G. C. Mead, editor, Poultry meat processing and quality. Woodhead Publishing, Cambridge. p. 232–257. doi: https://doi.org/10.1533/9781855739031.232.
Miettinen, M. K., L. Palmu, K. J. Björkroth, and H. Korkeala. 2001. Prevalence of Listeria monocytogenes in broilers at the abattoir, processing plant, and retail level. J. Food Protect. 64:994–999. doi: https://doi.org/10.4315/0362-028x-64.7.994.
Othman, N. M. 2007. Food safety in Southeast Asia: Challenges facing the region. Asian Journal of Agriculture and Development. 4:83–92. doi: https://doi.org/10.22004/ag.econ.166014.
Painter, J. A., R. M. Hoekstra, T. Ayers, R. V. Tauxe, C. R. Braden, F. J. Angulo, and P. M. Griffin. 2013. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 19:407–415. doi: https://doi.org/10.3201/eid1903.111866.
Pearce, R. A., D. J. Bolton, J. J. Sheridan, D. A. McDowell, I. S. Blair, and D. Harrington. 2004. Studies to determine the critical control points in pork slaughter hazard analysis and critical control point systems. Int. J. Food Microbiol. 90:331–339. doi: https://doi.org/10.1016/s0168-1605(03)00333-7.
Phan, T. T., L. T. L. Khai, N. Ogasawara, N. T. Tam, A. T. Okatani, M. Akiba, and H. Hayashidani. 2005. Contamination of Salmonella in retail meats and shrimps in the Mekong Delta, Vietnam. J. Food Protect. 68:1077–1080. doi: https://doi.org/10.4315/0362-028X-68.5.1077.
Pini, P. N., and R. J. Gilbert. 1988. The occurrence in the U.K. of Listeria species in raw chickens and soft cheeses. Int. J. Food Microbiol. 6:317–326. doi: https://doi.org/10.1016/0168-1605(88)90025-6.
Sánchez, M. X., W. M. Fluckey, M. M. Brashears, and S. R. McKee. 2002. Microbial profile and antibiotic susceptibility of Campylobacter spp. and Salmonella spp. in broilers processed in air-chilled and immersion-chilled environments. J. Food Protect. 65:948–956. doi: https://doi.org/10.4315/0362-028x-65.6.948.
Svobodová, I., G. Bořilová, R. Hulánková, and I. Steinhauserová. 2012. Microbiological quality of broiler carcasses during slaughter processing. Acta Vet. Brno. 81:37–42.
Ta, Y. T., T. T. Nguyen, P. B. To, D. X. Pham, H. T. H. Le, W. Q. Alali, I. Walls, D. M. A. Lo Fo Wong, and M. P. Doyle. 2012. Prevalence of Salmonella on chicken carcasses from retail markets in Vietnam. J. Food Protect. 75:1851–1854. doi: https://doi.org/10.4315/0362-028X.JFP-12-130.
Tibaijuka, B., B. Molla, G. Hildebrandt, and J. Kleer. 2003. Occurrence of Salmonellae in retail raw chicken products in Ethiopia. Berl. Munch. Tierarztl. 116:55–58.
USDA. 2014. Best management practices handbook: A guide to the mitigation of salmonella contamination at poultry hatcheries. http://www.poultryimprovement.org/documents/BestManagementPracticesHatcheries.pdf. (Accessed 15 December 2016.)
Uyttendaele, M., P. De Troy, and J. Debevere. 1999. Incidence of Salmonella, Campylobacter jejuni, Campylobacter coli, and Listeria monocytogenes in poultry carcasses and different types of poultry products for sale on the Belgian retail market. J. Food Protect. 62:735–740. doi: https://doi.org/10.4315/0362-028x-62.7.735.
Van, T. T. H., G. Moutafis, T. Istivan, L. T. Tran, and P. J. Coloe. 2007. Detection of Salmonella spp. in retail raw food samples from Vietnam and characterization of their antibiotic resistance. Appl. Environ. Microb. 73:6885–6890. doi: https://doi.org/10.1128/AEM.00972-07.
Vipham, J. L., M. M. Brashears, G. H. Loneragan, A. Echeverry, J. C. Brooks, W. E. Chaney, and M. F. Miller. 2012. Salmonella and Campylobacter baseline in retail ground beef and whole-muscle cuts purchased during 2010 in the United States. J. Food Protect. 75:2110–2115. doi: https://doi.org/10.4315/0362-028X.JFP-12-077.
Whyte, P., K. McGill, C. Monahan, and J. D. Collins. 2004. The effect of sampling time on the levels of micro-organisms recovered from broiler carcasses in a commercial slaughter plant. Food Microbiol. 21:59–65. doi: https://doi.org/10.1016/S0740-0020(03)00040-6.
Williams, M. S., E. D. Ebel, and H. D. Allender. 2015. Industry-level changes in microbial contamination on market hog and broiler chicken carcasses between two locations in the slaughter process. Food Control. 51:361–370. doi: https://doi.org/10.1016/j.foodcont.2014.11.039.
Yang, B., M. Xi, X. Wang, S. Cui, T. Yue, H. Hao, Y. Wang, Y. Cui, W. Q. Alali, J. Meng, I. Walls, D. M. Lo Fo Wong, and M. P. Doyle. 2011. Prevalence of Salmonella on raw poultry at retail markets in China. J. Food Protect. 74:1724–1728. doi: https://doi.org/10.4315/0362-028X.JFP-11-215.
Yang, X., J. Huang, Q. Wu, J. Zhang, S. Liu, W. Guo, S. Cai, and S. Yu. 2016. Prevalence, antimicrobial resistance and genetic diversity of Salmonella isolated from retail ready-to-eat foods in China. Food Control. 60:50–56. doi: https://doi.org/10.1016/j.foodcont.2015.07.019.
Zhao, C., B. Ge, J. De Villena, R. Sudler, E. Yeh, S. Zhao, D. G. White, D. Wagner, and J. Meng. 2001. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the Greater Washington, D.C., area. Appl. Environ. Microb. 67:5431–5436. https://doi.org/10.1128/AEM.67.12.5431-5436.2001.