Introduction
Genetic selection of broilers has resulted in significant improvements in growth rate and live performance, but modern fast-growing strains have contributed to an increase in skeletal muscle abnormalities, such as woody breast (WB) (Petracci et al., 2015). WB myopathy was first described by Sihvo et al. (2014) and is characterized by the severe hardening of the Pectoralis major muscle during growth to market weight, which affects up to 50% of broilers (Owens, 2014). Transcriptomic analysis of commercial fast- and slow-growth birds indicated that WB is a potentially polygenic, complicated syndrome that has similarities to neoplastic disorders (Hubert et al., 2018).
Microscopically, WB shows symptoms of inflammation, and the affected myofibers are usually replaced by fibrotic and adipose tissues (Huang and Ahn, 2018). WB myopathy is also associated with alterations in metabolism, decreased glycogen, increased fatty acid uptake, and increased mitochondrial oxidation of fatty acids, as well as increased hypoxia, oxidative stress, crosslinked collagen, and intracellular calcium concentration (Mutryn et al., 2015; Abasht et al., 2016; Soglia et al., 2016; Kuttappan et al, 2017; Zambonelli et al., 2017). WB has reduced technological and nutritional quality (poor water-holding capacity, texture, and perception), which decreased consumer acceptance due to visual as well as texture and flavor differences (Soglia et al., 2016; Petracci et al., 2019). WB has also been closely associated with nongenetic factors, including nutrition, environment, and management (Kuttappan et al., 2016). The use of greater amounts of synthetic amino acids (AAs) could contribute to the WB myopathy (Livingston et al., 2019). Therefore, an AA-reduced diet combined with different fast-growing strains could be evaluated in order to identify potential protein biomarkers that are associated with the WB myopathy. Specific proteomic signatures that are differentially abundant provide clues about biochemical processes and meat quality and allow for the determination of biological markers of meat quality defects and breast myopathies.
Proteomics has been applied to elucidate the role of diet in growth and meat quality in poultry and the protein biomarkers associated with poultry meat defects (Desai et al., 2016; Kuttappan et al., 2017; Cai et al., 2018). Kuttappan et al. (2017) compared the proteomic profiles of breast muscle tissue with no or minor and severe myopathic lesions and observed an increase in protein synthesis and cellular stress and a decrease in glycolytic potential in myopathic muscle. Likewise, Cai et al. (2018) evaluated the whole-muscle proteomic differences between normal breast (NB) and WB meat and observed that 8 proteins were expressed differentially between them, which indicated increased oxidative stress in WB meat compared to NB meat.
Therefore, this study was designed to compare the protein profiles between normal and woody chicken breast in order to identify potential protein biomarkers of WB myopathy and to determine the physiological response to protein abundance change as affected by broiler strains, diet, and postmortem time.
Materials and Methods
Egg incubation
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Mississippi State University (IACUC-16-542). Broiler eggs were acquired from 5 genetic strains (1–5) of 30-wk-old commercial breeder hens. All eggs were held for 4 d and then kept in incubators (Model PS 500, Jamesway Incubator Company Inc., Cambridge, Ontario, Canada). On day 12, eggs were candled, and unfertilized eggs were removed from incubators. Incubator dry and wet bulb temperatures were set at 37.5 ± 0.1°C and 28.9 ± 0.1°C, respectively.
Broilers, diet, and housing
After 21 d of incubation, a total of 1,280 mixed-sex broilers (256 birds/strain) were selected and raised in a chicken house in which 80 pens were evenly distributed in 8 blocks at the Poultry Research Farm at Mississippi State University. For each broiler strain, 256 birds were randomly assigned to 16 pens (2 pens/block); within each block, birds of each pen were fed with a control diet or an AA-reduced diet. Therefore, there were a total of 10 treatments (5 strains × 2 diets) within each block. The control diet was formulated to meet the highest recommended requirements, and the AA-reduced diet was formulated with 20% reduction of digestible lysine, total sulfur AAs, and threonine (Table 1). The temperature and lighting programs in the chicken house were monitored and regulated according to Zhai et al. (2016).
Feed ingredients composition and nutrient contents of a control diet (“Control”) with digestible amino acid (lysine, total sulfur amino acid, and threonine at the highest recommended level of 5 strains) and an amino-acid–reduced diet (“Reduced”) with these 3 digestible amino acids 20% lower than the recommended level during starter (day 0–14), grower (day 14–28), finisher (day 28–41), and withdrawal (day 41–60) feeding phases
| Starter | Grower | Finisher | Withdrawal | |||||
|---|---|---|---|---|---|---|---|---|
| Ingredients, % | Ctrl1 | Red1 | Ctrl | Red | Ctrl | Red | Ctrl | Red |
| Yellow corn | 54.3 | 62.3 | 55.5 | 68.1 | 67.2 | 73.3 | 69.2 | 76.6 |
| Soybean meal | 38.2 | 31.9 | 36.6 | 25.9 | 24.9 | 20.0 | 23.4 | 17.3 |
| Poultry grease | 2.50 | 1.15 | 3.60 | 1.67 | 3.50 | 2.37 | 3.50 | 2.30 |
| Dicalcium phosphate | 2.21 | 2.22 | 1.97 | 1.99 | 1.76 | 1.76 | 1.65 | 1.66 |
| Limestone | 1.28 | 1.31 | 1.17 | 1.22 | 1.10 | 1.12 | 1.05 | 1.08 |
| Salt | 0.34 | 0.34 | 0.34 | 0.34 | 0.34 | 0.35 | 0.35 | 0.35 |
| Choline chloride (60%) | 0.07 | 0.11 | 0.06 | 0.13 | 0.09 | 0.12 | 0.09 | 0.13 |
| L-lysine hydrochloride | 0.27 | 0.12 | 0.15 | 0.15 | 0.31 | 0.19 | 0.27 | 0.19 |
| DL-methionine | 0.32 | 0.18 | 0.26 | 0.11 | 0.28 | 0.16 | 0.23 | 0.13 |
| Premix2 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| L-threonine | 0.10 | 0.00 | 0.03 | 0.00 | 0.08 | 0.00 | 0.06 | 0.00 |
| Ronozyme | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Stafac3 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.00 | 0.00 |
| Sacox4 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.00 | 0.00 |
| Feed cost ($/ton) | 265 | 241 | 261 | 231 | 245 | 225 | 238 | 218 |
| Calculated composition | ||||||||
| Crude protein, % | 23.2 | 20.5 | 22.3 | 18.2 | 18.0 | 15.9 | 17.3 | 14.9 |
| Calcium, % | 0.96 | 0.96 | 0.87 | 0.87 | 0.78 | 0.78 | 0.74 | 0.74 |
| Available phosphorus % | 0.48 | 0.48 | 0.44 | 0.44 | 0.39 | 0.39 | 0.37 | 0.37 |
| M.E. (kcal/kg) | 3,009 | 3,009 | 3,100 | 3,100 | 3,199 | 3,199 | 3,225 | 3,225 |
| Digestible lysine, % | 1.28 | 1.024 | 1.15 | 0.92 | 1.02 | 0.82 | 0.95 | 0.76 |
| Digestible methionine, % | 0.67 | 0.50 | 0.60 | 0.41 | 0.57 | 0.42 | 0.51 | 0.38 |
| Digestible TSAA, % | 0.95 | 0.76 | 0.87 | 0.70 | 0.80 | 0.64 | 0.74 | 0.59 |
| Digestible threonine, % | 0.86 | 0.69 | 0.77 | 0.62 | 0.68 | 0.54 | 0.64 | 0.51 |
| Choline (ppm) | 1,800 | 1,800 | 1,700 | 1,700 | 1,500 | 1,500 | 1,450 | 1,450 |
| Sodium, % | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Chloride, % | 0.29 | 0.27 | 0.27 | 0.28 | 0.30 | 0.30 | 0.30 | 0.30 |
Amino acids in the control diet were at the highest recommend levels of digestible amino acid (lysine, TSAA, and threonine); reduced diet has digestible amino acid (lysine, TSAA, and threonine) 20% lower than the control diet.
Premix provided the following per kilogram of finished diet: retinyl acetate, 2.654 μg; cholecalciferol, 110 μg; DL-α-tocopherol acetate, 9.9 mg; menadione, 0.9 mg; vitamin B12, 0.01 mg; folic acid, 0.6 μg; choline, 379 mg; D-pantothenic acid, 8.8 mg; riboflavin, 5.0 mg; niacin, 33 mg; thiamine, 1.0 mg; D-biotin, 0.1 mg; pyridoxine, 0.9 mg; ethoxyquin, 28 mg; manganese, 55 mg; zinc, 50 mg; iron, 28 mg; copper, 4 mg; iodine, 0.5 mg; and selenium, 0.1 mg.
Stafac (Phibro Animal Health, Teaneck, NJ) provided 4.4% of virginiamycin to control enteric diseases.
Sacox (Huvepharma Inc., Peachtree City, GA) provided 13.2% of salinomycin sodium to prevent coccidiosis.
Ctrl = Control; M.E. = metabolizable energy; ppm = parts per million; Red = Reduced; TSAA = digestible total sulphur amino acid.
Harvest and sampling
At 8 wk of age, live broilers were evaluated by palpation for WB myopathy. One male bird was selected from each pen. Birds that yielded NB were selected from blocks 1–4, and birds that yielded WB were selected from blocks 5–8. Therefore, there were 4 male broilers with NB and 4 male broilers with WB within each treatment (strain × diet). A total of 80 birds were selected and euthanized using CO2 gas. The muscle (5–10 g) from the cranial portion of the right breast was collected and snap-frozen in liquid nitrogen after bleeding (0 min) and 15 min, 4 h, and 24 h post mortem. To confirm the manual palpation technique, the WB defects were evaluated at each time point according to Tijare et al. (2016) in which 0 = normal, 1 = slight, 2 = moderate, and 3 = severe. The live weight and carcass weight of each bird were measured. Three NB samples that were scored 0 and 1 at 0 min and three WB samples that were scored 2 and 3 at 0 min were selected for proteomic analysis.
pH measurement
A pH meter (Model Accumet 61a; Fisher Scientific, Hampton, NH) was used to measure breast fillet pH at death (pH0min) by inserting a pH probe (Model FlexipHet SS Penetration tip; Cole Palmer, Vernon Hills, IL) in the cranial portion, around 2.5 cm from the top of the Pectoralis major muscle.
Extraction and quantification of whole-muscle proteins
Frozen normal and woody broiler breast muscle tissues were ground in liquid nitrogen using a mortar and a pestle. Then, 200 mg of ground tissue was homogenized in 1.0 mL of ice-cold lysis buffer (8.3 M urea, 2 M thiourea, 2% 3-([3-cholamidopropyl]dimethylammonio)-1-propanesulfonate, and 1% dithiothreitol [DTT]) for 30 s using a homogenizer (FSH 500 homogenizer; Thermo Fisher Scientific, Waltham, MA). Homogenates were mixed vigorously for 2 h in iced water on a magnetic stirrer. Subsequently, the homogenates were centrifuged at 18,000g for 30 min at 4°C to remove debris. Supernatants were collected and quantified using the Bradford Assay (Bio-Rad, Hercules, CA) with bovine serum albumin (BSA) as the standard.
Two-dimensional gel electrophoresis
The protein extract (500 μg) of each chicken breast sample was mixed with Destreak rehydration buffer (GE Healthcare, Chicago, IL; added 1% DTT prior to use) and 0.5% ampholytes (GE Healthcare) to a final volume of 220 μl. Next, the mixture was centrifuged at 14,000g for 5 min to remove debris. Samples were then applied onto immobilized pH gradient (IPG) strips (pH 3 to 11 nonlinear, 11 cm; GE Healthcare) and covered with 2 mL mineral oil. The first-dimension isoelectric focusing was conducted using a Protean Isoelectric Focusing system (Bio-Rad, Hercules, CA) with the preset program, including 12 h of passive rehydration and a cumulative 35 kV·h of focusing. After focusing, the IPG strips were equilibrated twice for 15 min each in 2 mL of equilibration buffer I (6 M urea, 30% glycerol, 2.0% sodium dodecyl sulfate [SDS], 50 mM Tris HCl, 2.0% DTT; Bio-Rad, Hercules, CA) and 2 mL of Equilibration buffer II (6 M urea, 30% glycerol, 2.0% SDS, 50 mM Tris HCl, 2.5% iodoacetamide, and 0.001% Bromophenol blue; Bio-Rad, Hercules, CA). The equilibrated IPG strips were loaded onto 12.5% Criterion Precast gels (Bio-Rad, Hercules, CA) and overlaid with 0.75% agarose (Fisher Scientific, Atlanta, GA). The gels were submerged in tris-glycine electrophoresis buffer (25 mM Tris, 250 mM glycine, 0.1% SDS) and applied to an electronic field at 40 V for 20 min, 60 V for 20 min, and 100 V for 1 h. Gels were stained in Brilliant Blue G-Colloidal solution (Sigma-Aldrich, Milwaukee, WI) overnight and de-stained in 25% methanol (Fisher Scientific, Pittsburgh, PA) until the background stain was removed.
Gel image analysis
Gel images were captured using a Bio-Rad VersaDoc Model 3000 imaging system and analyzed with PDQuest software according to the protocol provided by Bio-Rad (Hercules, CA). Protein spots from the NB and WB meat gel images were detected and matched with the aid of landmarks using 6 gels from NB samples and 6 gels from WB samples. Spot intensities were normalized by expressing the relative quantity of each spot (parts per million [ppm]) as the ratio of individual spot quantity to the total quantity of each valid spot, and analyses were performed using qualitative, quantitative, and statistical modes. There were 300–400 matched protein spots detected in each comparison. In order to focus on myopathy-related proteins, only those spots that were found to be differentially abundant were extracted and identified by mass spectrometry. Protein spots on two-dimensional gels were considered differentially abundant between NB and WB samples when they exhibited a 1.5-fold or more intensity difference that was associated with a 5% statistical significance (P < 0.05) in the Student t test (Joseph et al., 2012).
Protein identification by mass spectrometry
The protein gel spots were excised and subjected to DTT reduction, iodoacetamide alkylation, and in-gel trypsin digestion according to Desai et al. (2014). The resulting tryptic peptides were extracted, concentrated, and subjected to shot-gun proteomics analysis as previously described in Kamelgarn et al. (2018). Liquid chromatography (LC)-tandem mass spectrometry (MS/MS) analysis was performed using a Linear Trap Quadropole-Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA) coupled with an Eksigent Nanoflex cHiPLC system (Eksigent, Dublin, CA) through a nano-electrospray ionization source. The peptide samples were separated with a reversed-phase cHiPLC column (75 μm × 150 mm) at a flow rate of 300 nL/min. Mobile phase A was 0.1% (v/v) formic acid in water, and B was 0.1% (v/v) formic acid in acetonitrile. A 50-min gradient condition was applied: initial 3% mobile phase B was increased linearly to 40% in 24 min and further to 85% and 95% for 5 min each before it was decreased to 3% and re-equilibrated. The mass analysis method consisted of one segment with 10 scan events. The first scan event was an Orbitrap mass spectrometry scan (300–1,800 m/z) with 60,000 resolution for parent ions followed by data-dependent MS/MS for fragmentation of the 9 most intense multiple charged ions using the collision-induced dissociation method.
Data analysis
Live weight, carcass weight, and pH data were analyzed in a randomized complete block design structure with a 5 × 2 × 2 factorial arrangement, considering strain (1–5), diet (control and AA-reduced), broiler breast (NB and WB), and their interactions as fixed effects; the animal as the experimental unit; and the pen as the block (1 bird/block). All analyses were carried out using the SAS General Linear Model procedure (version 9.4; SAS Institute Inc., Cary, NC). When significant differences (P < 0.05) occurred among treatments, Tukey’s honestly significant difference test was used to separate treatments (P < 0.05).
The LC-MS/MS data were submitted to a local mascot server for MS/MS protein identification via Proteome Discoverer (version 1.3; Thermo Fisher Scientific, Waltham, MA) against a custom database of Gallus (Chicken) proteins downloaded from National Center for Biotechnology Information. The parameters used in the MASCOT MS/MS ion search were as follows: trypsin digestion with a maximum of 2 miscleavages, cysteine carbamidomethylation, methionine oxidation, 10-ppm precursor ion, and 0.8-Da fragment ion mass tolerances. A decoy database was built and searched. Filter settings that determined false discovery rates were used to distribute the confidence indicators for the peptide matches. Peptide matches that passed the filter associated with the false discovery rate of 1% and 5% were assigned as high and medium confidence peptides, respectively.
Canonical pathways, diseases, and biofunctions related to the proteins that were differentially expressed between WB and NB were generated using Qiagen Ingenuity Pathway Analysis (IPA) (Qiagen, Venlo, Netherlands) and were compared against the Ingenuity Knowledge Base reference set. The P value for each subcategory was determined by dividing the number of differentially expressed protein genes (number of genes) within this subcategory by the total number of known genes in the subcategory that was present in the IPA software database “Query Ingenuity Knowledge Base.”
Results
Changes in technological properties of chicken breast
There were no three-way or two-way interactions among strain, diet, and breast myopathy (P > 0.05) for any traits evaluated. Thus, the main effects were presented for changes in live weight, carcass weight, and pH0min (Table 2). There was a strain effect on live weight (P = 0.0005) and carcass weight (P = 0.0004) but not on pH0min (P = 0.979). Strain 4 was heavier (P < 0.05) than strains 1, 2 and 5 but did not differ (P > 0.05) from strain 3 for both live and carcass weights. The pH0min of all strains were not different (P > 0.05), ranging from 6.34 to 6.38 (Table 2). In contrast, there was no diet effect (P > 0.05) on live and carcass weights, but there was an effect (P < 0.05) on pH0min when averaged over strain and breast myopathy (Table 2). Broilers that were fed a control diet had an average pH0min of 6.41, which was greater (P < 0.05) than birds that were fed an AA-reduced diet (6.33). When averaged over strain and diet, affected birds had greater live weight, carcass weight, and pH0min (P < 0.05) than normal birds. Compared with normal birds, affected birds were 7.9% heavier in live weight, 9.2% heavier in carcass weight, and 2.2% higher in pH0min (Table 2).
Live weight, carcass weight, and pH0min of 5 genetic strains of broilers that were fed with a control or an amino-acid–reduced diet with these 3 digestible amino acids 20% lower than the recommended level
| Treatment | Live weight/kg | Carcass weight/kg | pH0min | |
|---|---|---|---|---|
| Strain | Strain 1 | 4.39b | 4.01b | 6.37a |
| Strain 2 | 4.51b | 4.14b | 6.38a | |
| Strain 3 | 4.79a,b | 4.39ab | 6.34a | |
| Strain 4 | 4.96a | 4.59a | 6.38a | |
| Strain 5 | 4.37b | 3.99b | 6.37a | |
| SEM | 0.118 | 0.116 | 0.007 | |
| P value | 0.0005 | 0.0004 | 0.979 | |
| Diet | Control | 4.70a | 4.31a | 6.41a |
| AA-reduced | 4.51a | 4.13a | 6.33b | |
| SEM | 0.097 | 0.091 | 0.043 | |
| P value | 0.0534 | 0.0592 | 0.0481 | |
| Breast | Normal breast | 4.43b | 4.04b | 6.30b |
| Woody breast | 4.78a | 4.41a | 6.44a | |
| SEM | 0.180 | 0.186 | 0.069 | |
| P value | 0.0005 | 0.0002 | 0.0017 |
Means in a column sharing a common superscript were not different (P > 0.05).
AA = amino acid; pH0min = pH at death.
Proteomic differences between NB and WB samples
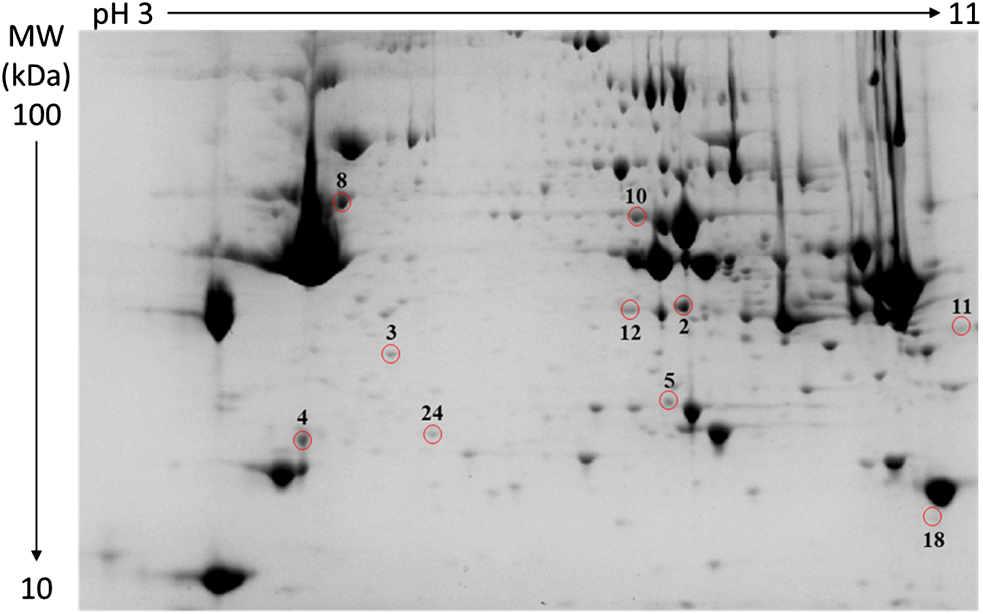
The following comparisons were made: (1) the proteome difference of NB and WB at 0 min from each strain of broilers fed with a control diet (comparisons 1–5: strains 1–5); (2) the proteome difference of NB and WB from the genetic strain 5 fed a control diet at 15 min, 4 h, and 24 h post mortem (comparisons 6–8); and (3) the proteome difference of NB and WB from the genetic strain 5 fed an AA-reduced diet (comparison 9). The analysis showed 4, 9, 1, 1, 10, 9, 9, 6, and 1 differentially abundant protein spots for comparisons 1–9, respectively (Table 3). 105400694445A typical gel image for comparison 5 is shown in Figure 1. Among these comparisons, there were a total of 27 different proteins with 24 of them identified. Twenty-two of these proteins were more abundant (fold change ratio > 1.5) in WB meat, and two proteins were less abundant (fold change ratio < 0.67) in WB meat, compared to NB meat (Table 3). Some proteins overlapped in several comparisons, and they were always consistent on the abundance direction (more or less abundant in WB meat) even though the fold change ratio differed (Table 3).
Proteins that were differentially abundant1 in woody breast compared to normal breast of broilers2
| Spot | Gene | Protein ID | Protein | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Overabundant In |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ALB | A0A1D5NW68 | Serum albumin | 1.72 | 1.79 | WB | |||||||
| 2 | ANXA2 | P17785 | Annexin A2 | 1.96 | 1.81 | 2.12 | WB | ||||||
| 3 | ANXA5 | P17153 | Annexin A5 | 1.81 | 2.12 | 2.12 | 1.6 | 2.16 | WB | ||||
| 4 | APOA1 | P08250 | Apolipoprotein A-1 | 2.3 | 2.37 | 2.58 | 2.39 | WB | |||||
| 5 | CA2 | P07630 | Carbonic anhydrase 2 | 0.65 | NB | ||||||||
| 6 | CFL2 | P21566 | Cofilin-2; muscle isoform | 2.31 | 2.00 | 1.82 | 1.7 | WB | |||||
| 7 | CKM | P00565 | Creatine kinase M-type | 1.55 | 1.88 | 2.15 | WB | ||||||
| 8 | DES | E1BZ05 | Desmin | 1.79 | 2.78 | 2.16 | WB | ||||||
| 9 | eEF1A2 | F1N9H4 | Elongation factor 1-alpha | 1.81 | 1.90 | WB | |||||||
| 10 | ENO3 | P07322 | Beta-enolase | 0.64 | 0.55 | NB | |||||||
| 11 | FHL1 | XP_004940737.1 | Four and a half LIM domains protein 1 | 212 | WB | ||||||||
| 12 | GPD1 | A0A3Q8WI14 | Glycerol-3-phosphate dehydrogenase 1 | 0.62 | 0.65 | NB | |||||||
| 13 | HSPB1 | Q00649 | Heat shock protein beta-1 | 3.15 | 2.34 | 2.65 | 2.65 | 2.62 | 2.24 | WB | |||
| 14 | LDHB | P00337 | L-lactate dehydrogenase B chain | 2.89 | WB | ||||||||
| 15 | PFKM | Q90YA3 | Phosphofructokinase | 1.55 | WB | ||||||||
| 16 | PGAM1 | Q5ZLN1 | Phosphoglycerate mutase-1 | 1.51 | WB | ||||||||
| 17 | PGM1 | XP_015146511.1 | Phosphoglucomutase-1 isoform X1 | 1.54 | WB | ||||||||
| 18 | PPIB | P24367 | Peptidyl-prolyl cis-trans isomerase B | 34.3 | WB | ||||||||
| 19 | TCP1 | XP_015153803.2 | T-complex protein 1 subunit gamma | 3.01 | WB | ||||||||
| 20 | TF | P68246 | Ovotransferrin | 1.64 | WB | ||||||||
| 21 | TNNI2 | P12620 | Troponin I, fast skeletal muscle | 2.36 | WB | ||||||||
| 22 | TNNT3 | P02789 | Troponin T, fast skeletal muscle | 2.85 | WB | ||||||||
| 23 | TUFM | P84172 | Elongation factor Tu, mitochondrial | 2.22 | WB | ||||||||
| 24 | UCHL1 | A1IMF0 | Ubiquitin carboxyl-terminal hydrolase | 2.92 | 84.9 | WB |
Comparison fold change was the protein abundance of WB vs. NB (fold change > 1.5 or < 0.67).
Nine comparisons between NB and WB were made and formatted as strain/sample time/diet: 1: strain 1/0 min/control, 2: strain 2/0 min/control, 3: strain 3/0 min/control, 4: strain 4/0 min/control, 5: strain 5/0 min/control, 6: strain 5/15 min/control, 7: strain 5/4 h/control, 8: strain 5/24 h/control, and 9: strain 5/0 min/reduced.
NB = normal breast; WB = woody breast.
Representative two-dimensional gel electrophoresis image of whole-muscle proteome of woody breast meat from strain 5 (at death) that were fed with a control diet. Differentially abundant protein spots between normal and woody breast samples (comparison 5 in Table 2) were presented: 2. annexin A2, 3. annexin A5, 4. apolipoprotein A-1, 5. carbonic anhydrase 2, 8. desmin, 10. beta-enolase, 11. four and a half LIM domains protein 1, 12. glycerol-3-phosphate dehydrogenase 1, 18. peptidyl-prolyl cis-trans isomerase B, 24. ubiquitin carboxyl-terminal hydrolase.
IPA canonical pathway analysis
The differentially expressed proteins in NB and WB are related to muscle structure, carbohydrate metabolism, transport and signaling, and cellular stress. Results from IPA revealed that the proteins that were differentially expressed between NB and WB were involved in biological pathways (P < 0.05). The summarized pathways and their corresponding protein genes are displayed in Table 4.
Ingenuity canonical pathways related to the proteins that were differentially abundant in broilers that showed woody breast myopathy as compared to controls
| Ingenuity Canonical Pathways | Molecules |
|---|---|
| Carbohydrate metabolism | |
| AMPK Signaling | CKM, PFKM |
| Creatine-phosphate Biosynthesis | CKM |
| GDP-glucose biosynthesis | PGM1 |
| Glucose and glucose-1-phosphate degradation | PGM1 |
| Glycerol Degradation I | GPD1 |
| Glycerol-3-phosphate Shuttle | GPD1 |
| Glycogenolysis | PGM1 |
| Glycolysis I | ENO3, PFKM, PGAM1 |
| HIF1α Signaling | LDHB |
| PFKFB4 Signaling Pathway | PFKM |
| Pyruvate Fermentation to Lactate | LDHB |
| Chaperones and cellular stress | |
| Cardiac Hypertrophy Signaling (Enhanced) | HSPB1 |
| Death Receptor Signaling | HSPB1 |
| ERK/MAPK Signaling | HSPB1 |
| IL-6 Signaling | HSPB1 |
| p38 MAPK Signaling | HSPB1 |
| Protein Ubiquitination Pathway | HSPB1, UCHL1 |
| Activation of IRF by Cytosolic Pattern Recognition Receptors | PPIB |
| Structural protein | |
| Actin Cytoskeleton Signaling | CFL2 |
| Axonal Guidance Signaling | CFL2 |
| Signaling by Rho Family GTPases | CFL2, DES |
| Transport and signaling | |
| Acute Phase Response Signaling | ALB, APOA1, TF |
| LXR/RXR Activation/FXR/RXR Activation | ALB, APOA1, TF |
| IL-12 Signaling and Production in Macrophages | ALB, APOA1 |
| Production of Nitric Oxide and Reactive Oxygen Species in Macrophages | ALB, APOA1 |
| BAG2 Signaling Pathway | ANXA2 |
| Osteoarthritis Pathway | ANXA2, ANXA5 |
| Calcium Transport I | ANXA5 |
ALB = serum albumin; AMPK = adenosine monophosphate–activated protein kinase; ANXA2 = annexin A2; ANXA5 = annexin A5; APOA1 = apolipoprotein A1; BAG2 = Bcl2-associated athanogene 2; CFL2 = cofilin-2, muscle isoform; CKM = creatine kinase M-type; DES = desmin; ENO3 = beta-enolase; ERK = extracellular signal-regulated kinase; FXR = farnesoid X receptor; GDP = guanosine diphosphate; GPD1 = glycerol-3-phosphate dehydrogenase 1; HIF1α =hypoxia-inducible factor 1-α; HSPB1 = heat shock protein beta-1; IL-12 = interleukin 12; IRF = interferon regulatory factor; LDHB = L-lactate dehydrogenase B chain; LXR = liver X receptor; MAPK = mitogen-activated protein kinase; PFKFB4 = fructose-2,6-biphosphatase 4; PFKM = 6-phosphofructokinase; PGAM1 = phosphoglycerate mutase 1; PGM1 = phosphoglucomutase-1; PPIB = peptidyl-prolyl cis-trans isomerase B; RXR = retinoid X receptor; TF = ovotransferrin; UCHL1 = ubiquitin carboxyl-terminal hydrolase.
IPA function and disease analysis
In order to investigate the functions and diseases that are related to the differentially expressed proteins, the 24 proteins from all comparisons were summarized, and the fold change ratio of overlapped proteins were averaged. This new dataset was submitted to IPA for analysis of diseases and biological functions. The results are presented in Table 5 and include the top 5 molecular and cellular functions and top 5 diseases and disorders along with P values. Results indicated that differentially expressed proteins between NB and WB were mainly associated with carbohydrate metabolism, cellular compromise, cellular assembly and organization, cellular function and maintenance, and cell death and survival (Table 5). IPA predicted decreased cell death through necrosis and apoptosis and increased cellular maintenance through ion homeostasis of cells and flux of Ca2+ in birds that had WB myopathy. These proteins have important functions and therefore may also be related to some diseases and disorders. The top 5 diseases included neurological disease, skeletal and muscular disorders, organismal injury and abnormalities, psychological disorders, and cancer (Table 5).
Top diseases and biofunctions associated with the proteins that were different in myopathic birds when compared to controls
| Top Diseases and Biofunctions | P value | Molecules |
|---|---|---|
| Molecular and cellular functions | ||
| Carbohydrate metabolism | 1.04E−02 to 3.77E−08 | 11 |
| Cellular compromise | 7.32E−03 to 1.43E−06 | 13 |
| Cellular assembly and organization | 8.36E−03 to 9.09E−06 | 15 |
| Cellular function and maintenance | 1.10E−02 to 1.03E−05 | 15 |
| Cell death and survival | 1.04E−02 to 1.33E−05 | 15 |
| Diseases and disorders | ||
| Neurological disease | 1.05E−02 to 4.96E−09 | 21 |
| Skeletal and muscular disorders | 1.02E−02 to 4.96E−09 | 19 |
| Organismal injury and abnormalities | 1.10E−02 to 1.02E−08 | 23 |
| Psychological disorders | 1.03E−02 to 1.02E−08 | 15 |
| Cancer | 1.10E−02 to 8.38E−08 | 23 |
Discussion
Broilers with different genetic background appear to respond to nutrition and environmental conditions differently; in other words, nutrition and environmental conditions may confer similarities and differences in protein expression and biofunctions for genetically different broiler strains. In this manuscript, although samples were collected from chicken breast of different genetic strains fed on different diets or at different postmortem time, the focus will be comparing the proteomic differences between NB and WB. The time and genetic effects on the proteomic differences will be discussed in other manuscripts.
Carbohydrate metabolism in response to WB myopathy
Beta-enolase and glycerol-3-phosphate dehydrogenase 1
Both beta-enolase (ENO3) and glycerol-3-phosphate dehydrogenase 1 (GPD1) were less abundant in WB when compared to NB for strains 2 and 5 (Table 2); these two strains had higher WB incidence compared with others (Zhang et al., 2018). None of these proteins were differentially abundant between NB and WB from strains 1 and 3. ENO3 is an essential glycolytic enzyme that catalyzes the reversible dehydration of 2-phosphoglycerate to the high-energy intermediate phosphoenolpyruvate. The decreased abundance of ENO3 indicated decreased glycolytic activity and increased pH in WB. The WB in the current study did have a higher pH0min than NB for all broiler strains (Table 2). Due to the nature of its glycolytic activity, levels of ENO3 in skeletal muscle are dependent on fiber type. Levels of ENO3 are higher in fast-twitch glycolytic type IIB fibers (the most glycolytic muscle) followed by IIX, IIA, and I (Keller et al., 2000). Chicken breast mainly consists of type IIB or anaerobic fibers. The lesser abundance of ENO3 in WB was also indicative of a potential fiber-type switching, from fast-twitch glycolytic fibers to slow-twitch oxidative fibers in response to WB myopathy (Soglia et al., 2016).
GPD1 catalyzes the reversible redox conversion of dihydroxyacetone phosphate to glycerol-3-phosphate in the glycerol-3-phosphate shuttle pathway (D’Alessandro et al., 2011). Glycerol-3-phosphate can be a substrate either in glycerophospholipid metabolism or the glycolysis cascade. Therefore, GPD1 serves as a link between carbohydrate and lipid metabolism. Upon entering the glycolysis cascade, glycerol-3-phosphate is converted to glyceraldehyde-3-phosphate and follows the glycolysis cascade until pyruvate is formed (Liu et al., 2018). Lower abundance of GPD1 in WB may limit the glyceraldehyde-3-phosphate supply to the glycolysis pathway and decrease glycolytic activity. GPD1 is also involved in maintaining the redox potential across the inner mitochondrial membrane (Harding et al., 1975). Reduced GPD1 activity in WB of strains 2 and 5 indicates a lower glycolytic activity and an impaired redox balance. This result is different than that of Zambonelli et al. (2017), who reported greater abundance of GPD1 in abnormal chicken breast compared to normal chicken breast.
Phosphoglucomutase-1
WB from strain 4 at 0 min had increased phosphoglucomutase-1 (PGM1) activity (Table 3) compared to NB. IPA indicated upregulation of guanosine diphosphate-glucose biosynthesis, glucose and glucose-1-phosphate degradation, and glycogenolysis pathways in WB when compared to NB (Table 4). Increased PGM1 activity favors the breakdown of glycogen and the production of glucose-6-phosphate (Anderson et al., 2014). However, for strain 4, PGM1 was the only glycolytic enzyme that was more abundant in WB compared to NB meat. Other glycolytic enzymes were not differentially abundant (Table 3). Therefore, the glycogenolysis product—glucose-6-phosphate—might be utilized in other pathways. Abasht et al. (2016) investigated the metabolite abundance in broilers affected by the WB myopathy and found reduced glycogen content and glycolytic activity, which may be due to the change in glucose utilization rather than glucose availability.
Creatine kinase M-type
Creatine kinase M-type (CKM) was overabundant in WB from strain 2 at 0 min and from strain 5 at 15 min and 4 h post mortem (Table 3). In fast-twitch glycolytic skeletal muscle, creatine can be rapidly and reversibly phosphorylated to phosphocreatine, which serves as a high-energy reservoir and maintains the adenosine triphosphate (ATP)/adenosine diphosphate ratio (Lefaucheur, 2010). Phosphocreatine helps replenish ATP when there is a high demand in cells and tissues, such as in rapid-growing broiler strains 2 and 5. Therefore, CKM is involved in cellular energy homeostasis. Under anaerobic conditions (for example, in postmortem muscles), phosphocreatine can generate ATP during a short period when oxidative phosphorylation is not available, which delays the lactate production from pyruvate and pH decline (Lefaucheur, 2010). This may be why WB meat has a greater pH0min than NB meat. Desai et al. (2016) reported greater abundance of CKM in Pale, Soft, and Exudative–like meat, relating its activity to a lower pH in chicken breast. In the current study, the greater abundance of CKM in WB might be a physiological reaction in response to a higher demand for ATP in affected birds under stress or hypoxia.
L-lactate dehydrogenase B chain
L-lactate dehydrogenase B chain (LDHB) was uniquely more abundant at 15 min post mortem in WB meat from strain 5 compared to NB meat. The greater abundance of LDHB is associated with unregulated pyruvate-fermentation-to-lactate pathway and the hypoxia-inducible factor 1-α signaling pathway (Tables 3 and 4). It is logical that LDHB was upregulated in WB 15 min after death but not at 0 min. Under anaerobic conditions, muscle can utilize LDHB to convert pyruvate to lactate. Soglia et al. (2016) and Zambonelli et al. (2017) reported higher LDHB abundance and higher pH in myopathic chicken muscle. This indicates that there might not be an increase in the transformation of pyruvate into lactate, as normally expected under hypoxic conditions. Berri et al. (2007) stated that postmortem lactate production can be limited due to low glycogen content in the muscle. The LDHB reaction uses 2 electrons, 1 nicotinamide adenine dinucleotide proton, and 1 cytosol proton to promote the reduction of pyruvate to lactate. This consumption of protons slows down acidosis. The greater abundance of LDHB in WB muscle may be an indicator of hypoxic conditions for live birds since hypoxia-inducible factor 1-α usually accumulates under hypoxic conditions (Malila et al., 2019).
ATP-dependent 6-phosphofructokinase and phosphoglycerate mutase 1
ATP-dependent 6-phosphofructokinase (PFKM) and phosphoglycerate mutase 1 (PGAM1) were uniquely more abundant in WB than NB meat from broiler strain 5 at 4 h post mortem (Tables 3 and 4). PFKM catalyzes the phosphorylation of fructose 6-phosphate to fructose 1,6-bisphosphate. This reaction is unidirectional and drives metabolism towards glycolysis because a different enzyme, fructose bisphosphatase, is required to catalyze the reverse reaction. PFKM is a key regulator of glycolysis that is activated when the ATP level is low and the adenosine monophosphate level is high. ATP production dramatically decreases post mortem, signaling a need to activate PFKM and consequently glycolysis (Scheffler and Gerrard, 2007). The greater abundance of PFKM is related to upregulation of the adenosine monophosphate–activated protein kinase signaling pathway and the fructose-2,6-biphosphatase 4 (PFKFB4) signaling pathway (Table 4). Adenosine monophosphate–activated protein kinase activates PFKM, which adds one phosphate on fructose-6-phosphate to produce fructose 1,6-bisphosphate and fructose 2,6-bisphosphate to rapidly activate glycolysis. The level of fructose 2,6-bisphosphate is controlled by PFKFB4, which contributes to an elevated rate of glycolysis for energy production (Atkinson, 1977). Glycolytic muscles are expected to have a greater abundance of PFKM early post mortem since glycolysis is in full swing, but it could be drastically decreased as time after slaughter increases once rigor mortis has begun (Scheffler and Gerrard, 2007).
PGAM1 is a glycolytic enzyme that catalyzes the interconversion of 3-phosphoglycerate to 2-phosphoglycerate with 2,3-bisphosphoglycerate as a cofactor in glycolysis (Wang et al., 2018). This protein is involved in glycolysis and a key catalytic step in anaerobic ATP regeneration in skeletal muscle. The greater abundance of PGAM1 suggests that myopathic WB meat requires more metabolic energy for muscle maintenance. Cai et al. (2018) also reported a 6.32-fold greater abundance of PGAM (abbreviated by Cai et al. as “PGM”) in 24-h–post-mortem WB meat, suggesting that the glycolytic pathway might be upregulated in response to need for ATP. Compared with 15 min post mortem, there was more intense glycolysis and pH decline after 4 h, indicating greater ATP needs during rigor mortis. At 24 h post mortem, glycolytic enzymes were not differentially abundant between NB and WB meat (Table 3), which indicates that rigor mortis had already been completed. Therefore, the abundance of PGAM1 is not only related to the myopathy but also the muscle biochemistry as muscle is converted to meat.
Chaperones and oxidative stress
Chaperones assist in the folding and refolding of proteins to prevent aberrant folding and aggregation during both homeostasis and stress (Hartl et al., 2011).
Heat shock protein beta-1
Heat shock protein beta-1 (HSPB1), also known as hsp27 or stress protein, is upregulated during oxidative damage in order to scavenge reactive oxygen species (ROSs), maintain the intracellular redox homeostasis, and protect against apoptosis (Arrigo et al., 2007). Compared to NB, HSPB1 was more abundant in WB from strains 1 and 2 at 0 min, strain 5 post mortem, and strain 5 that was fed a reduced diet (Table 3), indicating greater oxidative stress in WB. This is confirmed by the upregulated pathway production of nitric oxide and ROSs due to the greater abundance of ALB and apolipoprotein A1 (APOA1) in WB from strains 1 and 2, and downregulated death receptor signaling (Table 4). Additionally, greater abundance of HSPB1 is related to unregulated p38 and extracellular signal-regulated kinase/mitogen-activated protein kinase signaling pathways (Table 4). Furthermore, HSPB1 modulates the protein ubiquitination pathway and is essential for proper protein folding and cytoskeletal integrity (Mounier and Arrigo, 2002). HSPB1 is responsible for inhibiting ROSs and consequently affecting myofibrillar degradation, since a synergy exists between ROSs and caspase-3 that directly impacts calpain activity and myofibrillar degradation (Lana and Zolla, 2016). The increased HSPB1 activity might be indicative of a collapsed F-actin cytoskeleton or degraded myofibrillar structure since the chaperones are the first defense against misfolded, aggregation-prone proteins and aberrant protein interactions (Arrigo et al., 2007). Finally, HSPB1 is related to interleukin-6 signaling and cardiac hypertrophy signaling pathways, indicating that it is associated with inflammation and cardiovascular disease. WB myopathy histologically exhibits hypertrophy, a wide variation in fiber size, degenerative and regenerative changes in the myofibers, and various levels of fibrosis with occasional infiltration by inflammatory cells (Kawasaki et al., 2018). Several other studies have indicated that heat shock protein was overabundant in WB samples compared to NB and reported that WB had inflammation tissues and hypertrophic myocytes due to oxidative stress and hypoxia (Mutryn et al. 2015; Kuttappan et al., 2017; Zambonelli et al., 2017; Malila et al., 2019).
Elongation factor 1-alpha and mitochondrial elongation factor Tu
Elongation factors belong to GTPase and participate in the polypeptide elongation process of protein synthesis through translation in the ribosome (Liu et al., 2010). In eukaryotic cells, elongation factors Elongation Factor 1-Alpha (eEF1A1) and mitochondrial elongation factor Tu (TUFM) are localized in cytoplasm and mitochondria. Compared to NB meat, eEF1A1 was more abundant in WB from strain 5 at 15 min post mortem, while TUFM was more abundant in WB from strain 5 at 24 h post mortem (Table 3). EF1A1 and TUFM may have been upregulated in response to needs for protein synthesis or repair of misfolded proteins during growth. The differential abundance of elongation factors post mortem might reflect the antemortem status of collected tissue. Although we observed WB phenotype in the cranial region, the attributes could be localized as indicated by our experience on WB sensory evaluation and the presence of localized rigidity described by Sihvo et al. (2018). In addition, both eEF1A and TUFM participate in the entire process of the heat shock response from transcription through translation (Vera et al., 2014). For example, eEF1A1 binds to the 3′ untranslated region of HSP70 and regulates the heat shock response, making it a target for the treatment of many pathological conditions associated with heat shock response deregulation (Vera et al., 2014). In general, the most direct pathway to activation of programmed cell death is the activation of receptor proximal initiator caspases. Other organelle-specific initiators of cell death include endoplasmic reticulum (ER), which can sense perturbations in Ca2+ homeostasis or protein folding and trigger apoptosis by the unfolded protein response and the ER-specific protease (Nakagawa et al., 2000). Talapatra et al. (2002) reported that eEF1A1 expression does not protect from nuclear or death-receptor–initiated apoptosis but does prevent death from ER stress, suggesting a role for eEF1A1 as a regulator of apoptosis.
Peptidyl-prolyl cis-trans isomerase B
Peptidyl-prolyl cis-trans isomerase B (PPIB) was 34.3 times more abundant in WB than NB for strain 5, implying that WB may have higher concentrations of type I collagen and disordered proteins. Collagen content is greater in WB than NB (Soglia et al., 2016). PPIB is localized to the ER and upregulated in response to ROS accumulation and ER stress to maintain redox homeostasis (Blair et al., 2015). IPA indicated that the greater abundance of PPIB in WB activated interferon regulatory factor by cytosolic pattern recognition receptors (Table 4), which results in an innate immune response against disease, viruses, and bacteria.
T-complex protein 1
T-complex protein 1 (TCP1) plays a role in protein folding or refolding of a variety of proteins in an ATP-dependent manner, and the 2 main substrates are actin and tubulin (Berger et al., 2018). TCP also caused cellular growth and proliferation according to studies in human cell lines and mouse tissues (Kubota et al., 1992; Coghlin et al., 2006). TCP1 was overabundant in WB of strain 5 at 15 min post mortem (Table 3). The greater abundance of TCP1 has been correlated to the development of several types of cancer (Cui et al., 2015; Li et al., 2017).
Ubiquitin carboxyl-terminal hydrolase
Ubiquitin carboxyl-terminal hydrolase (UCHL1) is more abundant in WB than NB from strain 5 at 0 and 15 min (Table 3). UCHL1 is a chaperoning client of 2-Cys peroxiredoxins (Lee et al., 2018). UCHL1 is primarily expressed in neuronal tissues and is sometimes upregulated in skeletal muscles in disease conditions or during injury (Gao et al., 2017). It was proven in a mouse study that UCHL1 affects skeletal muscle function through the neuro-control of muscles (Chen et al., 2010). UCHL1 contributes to early processes of myogenesis through promoting myoblast proliferation and inhibiting cell differentiation (Gao et al., 2017).
Structural proteins
Cofilin-2, muscle isoform
Cofilin-2, muscle isoform (CFL2) was more abundant in WB than NB meat from strains 2 and 3, as well as strain 5 at 15 min and 4 h post mortem (Table 3). CFL2 is a skeletal-muscle–specific protein localized to the thin filament, cytoplasm, and nucleus that controls actin polymerization and depolymerization in a pH-sensitive manner, with maximal depolymerization at pH 8.0 that is almost nonexistent at pH < 7.0 (Papalouka et al., 2009). This indicates that the normal postmortem pH decline due to lactic acid accumulation will favor actin polymerization and sarcomere structure contraction, which contributes to improved tenderness (Franco et al., 2015). CFL2 is a ubiquitous actin-binding component for actin filament reorganization, which is an indicator of cellular repair in response to muscle damage. The greater abundance of CFL2 in WB indicates that it might participate in cellular and muscle repair through cell motility and rearrangement of actin filaments due to upregulated actin cytoskeleton signaling and axonal guidance signaling (Table 4). Birds that are affected by WB attempt repair and regeneration of cytoskeleton muscle and therefore are probably the result, not the primary cause, of WB myopathy (Mutryn et al., 2015).
Desmin
Desmin (DES) comprises a set of intermediate filaments that are essential for myofibrillar integrity and maintenance of overall cellular integrity and organization of skeletal muscle cells. DES was overabundant in WB from strain 1 and 5 at death and from strain 5 at 4 h post mortem (Table 3). Regarding the live muscle, the increased abundance of these proteins indicates interrupted myofibrillar integrity in affected birds, which is also supported by the increased activity of stress proteins in this study that decrease apoptosis and increase protein synthesis (Arrigo et al., 2007). It is well known that DES is degraded post mortem, resulting in an improved tenderness (Tomaszewska-Gras et al., 2011). In this study, DES was less abundant in NB than WB at 4 h post mortem, indicating that NB might be more tender than WB. Byron et al. (2020) measured the shear force of cooked chicken breast over refrigerated storage time and reported that shear force values decreased over refrigerated storage time for NB but not WB. According to Tomaszewska-Gras et al. (2011), the DES degradation process in chicken muscles starts within 3 h post mortem and is related to an increased myofibrillar fragment index, a measure of the average length of myofibrils that is related to tenderness. Soglia et al. (2016) also reported greater abundance of DES and higher shear force values in WB meat compared to NB.
Four-and-a-half LIM protein 1
Four-and-a-half LIM protein 1 (FHL1) was 212 times more abundant in WB than NB from strain 5 at death (Table 3). FHL1 belongs to the FHL protein family, which is associated with muscle hypertrophy. Skeletal muscle hypertrophy is characterized by enhanced transcription/translation, which results in increased protein synthesis and reduced protein breakdown (Boonyarom and Inui, 2006). FHL1 is localized to the I-band, M-line, and sarcolemma, colocalizing with myosin-binding protein C at the sarcolemma in intact skeletal muscle. The greater abundance of FHL1 impaired Z-line and myosin thick filament assembly in a cell line study (McGrath et al., 2006). FHL1 regulates body mass and increases muscle fiber size and oxidative slow-fiber–type expression, which leads to increased muscle strength and endurance in mice (Cowling et al., 2008). The reduced glycolytic activity confirmed the muscle fiber-type switch from glycolytic to oxidative. Windpassinger et al. (2008) also noticed a greater abundance of FHL1 in myopathic muscles, which has been related to DES accumulation in the muscle fibers.
Troponin I, fast skeletal muscle and troponin T, fast skeletal muscle
Troponin is a three-subunit complex: Ca2+ binding troponin C (TnC), inhibitory troponin I (TnI), and tropomyosin-binding troponin T (TnT). Troponin I, fast skeletal muscle and Troponin T, fast skeletal muscle are genes that are expressed in fast skeletal muscle for TnI and TnT, respectively. They were overabundant in strain 5 at 4 h and 24 h post mortem (Table 3), indicating that less Troponin I, fast skeletal muscle and Troponin T, fast skeletal muscle were degraded in WB than in NB post mortem. Desai et al. (2016) and Silva et al. (2019) reported that TnT and TnI were more abundant in tough meat. TnT and TnI are a regulatory protein complex that is central to muscle contraction in skeletal and cardiac muscles. According to Silva et al. (2019), the progress of degradation of the myofibrillar TnT subunit seems to be a good predictor of tenderization during normal postmortem aging of beef.
Transport and signaling proteins
ALB and APOA1
ALB and APOA1 were more abundant in WB than NB from strains 1 and 2 at 0 min; APOA1 was also more abundant in strain 5 at 0 min and 15 min post mortem (Table 3). IPA indicated that APOA1 and ALB are involved in several canonical pathways, including atherosclerosis signaling, liver X receptor/retinoid X receptor activation, acute phase response signaling, and production of nitric oxide and ROSs (Table 4). ALB is the most abundant protein in blood plasma and is primarily synthesized by the liver. It regulates the colloidal osmotic pressure of blood and binds and transports fatty acids, cholesterol, and some ions (copper, zinc, and calcium) during blood circulation. ALB is also an antioxidant that protects against peroxidative damage (Evans, 2002). ALB was more abundant in the current study in response to the upregulated pathway production of nitric oxide and ROSs in WB compared to NB. Similarly, Cai et al. (2018) reported that ALB was overabundant in WB in comparison to NB. Mutryn et al. (2015) also reported upregulation of the ALB RNA gene in WB. Merlot et al. (2014) proposed that when exposed to cellular stress, ALB is absorbed by normal cells at low levels and tumor cells at high levels due to their metabolic rate. In this study, increased cellular repair and protein synthesis may stimulate the WB-affected birds to express more ALB in the circulation to transport molecules.
APOA1 participates in the reverse cholesterol transport from tissue to liver to maintain cholesterol homeostasis. It assists in the removal of cholesterol from white blood cells within artery walls. Therefore, APOA1 helps in preventing further progression of atherosclerosis by inhibiting fat accumulation within white blood cells (Tangirala et al., 1999). In addition, as a negative acute-phase reactant, APOA1 is anti-inflammatory towards acute and chronic inflammatory diseases (Mangaraj and Nanda, 2016), as indicated by activated liver X receptors/retinoid X receptors (Table 4) that are involved in the regulation of lipid metabolism, inflammation, and catabolism of cholesterol to bile acid. Moreover, APOA1 increased glucose disposal in skeletal muscle by activating an IR/IRS-1/PI3K/Akt/AS160 signal transduction pathway in human skeletal muscle cells (Tang et al., 2019). In this study, the greater abundance of APOA1 in WB meat could be related to its physiopathology since lipidosis is a characteristic of the WB myopathy (Soglia et al., 2016; Papah and Abasht, 2019).
Annexins A2 and A5
The annexins are structurally related Ca2+-binding proteins that bind to phospholipids in a calcium-dependent manner. Annexin A2 (ANXA2) was more abundant in WB than NB from strain 5 at 0 min and 4 and 24 h post mortem (Table 3). Annexin A5 (ANXA5) was more abundant in WB than NB of strain 2 at 0 min and strain 5 at 0 min and post mortem (Table 3). The greater abundance of ANXA2 and ANXA5 in WB are indicative of necrosis. Bouter et al. (2011) reported that ANXA5 proteins could bind to torn membrane edges of mouse perivascular cells and form a two-dimensional array, which prevents the expansion of tissue and promotes membrane resealing. Demonbreun et al. (2019) found that an actin-dependent annexin complex (A1, A2, A5, and A6) mediates plasma membrane repair of lesions and tears in muscle.
At the cell surface, ANXA2-p11 complex stimulates plasmin production and results in fibrinolysis and angiogenesis, which might contribute to surface hemorrhaging that occurs in WB muscle. The Bcl-2-associated athanogene 2 signaling pathway was upregulated due to greater abundance of ANXA2 in WB (Table 4). Bcl-2-associated athanogene 2 regulates pro-cathepsin B/ANXA2 complex formation and enhances the secretion of pro-cathepsin B, which favors metastasis of carcinoma. Upregulated ANNXA2 has been associated with colorectal cancer, gastric cancer, lung cancer, neurological cancer, etc. (Christensen et al., 2018). IPA indicated upregulation of calcium transport due to the increased ANXA5 presence in WB (Table 4). ANXA5 can form Ca2+-permeable channels in nonoxidized membranes but also mediate peroxide-induced Ca2+ influx across the phospholipid membranes of chicken DT40 cells (Gerke and Moss, 2002). ANXA5 acts as an antithrombotic arm of blood coagulation due to its capacity to bind calcium to phospholipids (Reutelingsperger et al., 1988). Mutryn et al. (2015) reported downregulated coagulation pathways in WB-affected tissue with signs of inflammation. Blood coagulation starts with vascular damage and inflammation, upregulation of anticoagulating (the current study), and downregulation of coagulation (Mutryn et al., 2015), which may be the cellular repair mechanism that affected birds use to fight against inflammation. In addition, ANXA5 has been found to modulate the immune system in vivo by inhibiting phagocytosis of apoptotic and necrotic cells (Munoz et al., 2007).
Carbonic anhydrase 2
Carbonic anhydrase 2 (CA2) was 1.5-fold downregulated in WB of strain 5 at 0 min, but not differentially abundant in other broiler species (Table 3). This may indicate that affected birds may have more CO2 and lower pH due to the weak ability to remove CO2. However, it may also indicate that less CA2 is needed due to less CO2 production since less oxygen is reaching WB tissue (Geers and Gros, 2000). It is known that CO2 produced within skeletal muscle must be eliminated via lung ventilation, but there are several steps to transport it to the lungs (Geers and Gros, 2000). Carbonic anhydrase assists CO2 transportation through accelerating the hydration/dehydration reaction between CO2, HCO3−, and H+. In skeletal muscle, lactic acid and 3 forms of CO2 contribute to H+ during exercise. Therefore, downregulated CA2 and glycolytic enzymes (ENO3) contribute to the high pH in WB.
Ovotransferrin
Ovotransferrin (TF) was overabundant in WB from strain 5 after 24 h post mortem (Table 3). TF has regulatory roles in chicken embryo development, antibacterial and antiviral characteristics, immune response, and antioxidation due to superoxide radical-scavenging activity (Chen et al., 2017). Cooper et al. (2019) observed a greater abundance of TF in chicken breast from animals that were under stress during growth. Thus, the greater abundance of TF in WB meat could be related to the increase in oxidative stress, as indicated for several chaperones that were more abundant in WB than NB meat.
Differences in protein abundances from WB and WB
Genetic effect
The 5 commercially available genetic strains that were fed with the control diet were included in this study for comparisons 1–5. Strains 1 and 2 share similar genetic backgrounds, and strains 4 and 5 are from similar genetic backgrounds. Strains 2 and 5 had a traditionally higher WB incidence followed by strains 1, 3, and 4 (Zhang et al., 2018). Strain 4 had the least percentage of severe WB (Zhang et al., 2018) but yielded the heaviest birds among all broiler strains (Table 2), indicating that the WB incidence was not always associated with bird weight. The differences in protein abundance between NB and WB were highly variable among genetic broiler strains for comparisons 1–5, indicating that the underlying mechanism for WB development may vary among broiler strains. Strains 1 and 2 shared 3 protein biomarkers, ALB, APOA1, and HSPB1 (Table 3). However, strains 4 and 5, which have the similar genetic background, did not share any protein biomarkers. Strain 4 only had one differentially abundant protein, PGM1, between NB and WB, while 10 proteins were differentially abundant for strain 5. CFL2 was the only protein that was overabundant in WB for strain 3. Strains 2 and 5, which are rapid growth strains, had more protein abundance differences than other strains and shared 4 protein biomarkers, including ANXA5, APOA1, ENO3, and GPD1. Overall, the development of WB myopathy is attributed to the changes in glycolytic enzymes, chaperone proteins, structural proteins, and transport proteins although it is not clear which proteins initiate the myopathy. In fact, the proteins identified in this study may interact in the development of the myopathy. For example, HSPB1 regulates protein folding, therefore the upregulation of HSPB1 under stress may have an impact on cytoskeletal structure in which structure and transport proteins are involved. This chain of reaction may lead to the development of WB or other myopathies. The threshold of protein abundance difference in this study was set as 1.5-fold. Therefore, those proteins that were below this ratio were not selected for protein identification. This may partially contribute to the nonconsistency of protein biomarkers among strains.
Nutritional effect
As mentioned earlier, 10 proteins were differentially abundant in WB compared with NB from strain 5 that were fed with the control diet (comparison 5; Table 3). However, only one protein (HSPB1) was differentially abundant (P < 0.05) when broiler strain 5 was fed with the AA-reduced diet (Table 3). This protein was not on the list of differentially abundant proteins for strain 5. Reducing AA intake in the diet decreased the bird weight, breast weight, and WB incidence after 8 wk of growth (Zhang et al., 2018) as well as the number of protein biomarkers for WB development. The birds that received the AA-reduced diet are anticipated to have less oxidative stress compared with birds that were fed with the control diet, which may partially explain the minimal protein profile differences for AA-reduced birds. However, minimal difference also indicates that there may be additional factors other than genetics or nutrition that trigger the WB myopathy.
Postmortem effect
The chicken breast samples collected at death (0 min) were considered as a reflection of live muscle. At 0 min, strain 5 that produced WB showed decreased glycolytic activity (downregulated ENO3 and GPD1) and a higher pH0min value compared with NB. Early post mortem, the cells in WB samples worked harder than NB to generate ATP in response to a greater demand for oxygen, which can be seen in the unregulated transport proteins, stress proteins, and structural proteins. Several proteins remained overabundant in WB after 4 h post mortem, including ANXA5, CFL2, CKM, and HSPB1. The glycolytic activity in WB was predicted to be higher than NB after 4 h post mortem, which may indicate that NB had already completed the rigor mortis, but WB had not. At 24 h post mortem, no difference in glycolytic enzymes between NB and WB were found, which might be due to the completion of rigor mortis. However, proteins ANXA5 and HSPB1 were overabundant in WB.
Conclusions
WB and NB had different proteome profiles, and the difference was dependent on genetic strain, diet, and postmortem time. The proteomic analysis in this study identified 7 glycolytic proteins, 6 stress proteins, 5 structural proteins, and 6 transport proteins that were more abundant in WB than NB. It is suggested that stress, structural proteins, and transport proteins were more abundant in WB than NB although not all proteins were consistently present in each comparison. Among them, ANXA2, APOA1, CFL2, and HSPB1 were present in more than 4 comparisons and were considered as major contributors to WB myopathy. These differentially abundant proteins in WB are mainly related to oxidative stress, hypoxia, muscle damage, and high energy demand, indicating that WB-affected birds grow faster and bigger and therefore have a higher demand for oxygen, energy, and metabolism.
Acknowledgements
The USDA-NIFA Foundation Program (2017-67017-26473) funded this research.
Literature Cited
Abasht, B., M. F. Mutryn, R. D. Michalek, and W. R. Lee. 2016. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS One. 11:e0153750. https://doi.org/10.1371/journal.pone.0153750.
Anderson, M. J., S. M. Lonergan, and E. Huff-Lonergan. 2014. Differences in phosphorylation of phosphoglucomutase 1 in beef steaks from the longissimus dorsi with high or low star probe values. Meat Sci. 96:379–384. https://doi.org/10.1016/j.meatsci.2013.07.017.
Arrigo, A. P., S. Simon, B. Gibert, C. Kretz-Remy, M. Nivon, A. Czekalla, D. Guillet, M. Moulin, C. Diaz-Latoud, and P. Vicart. 2007. Hsp27 (HspB1) and αB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 581:3665–3674. https://doi.org/10.1016/j.febslet.2007.04.033.
Atkinson, D. E. 1977. Cellular energy metabolism and its regulation. Academic Press, New York, NY.
Berger, J., S. Berger, M. Li, A. S. Jacoby, A. Arner, N. Bavi, A. G. Stewart, and P. D. Currie. 2018. In vivo function of the chaperonin TRiC in α-actin folding during sarcomere assembly. Cell Rep. 22:313–322. https://doi.org/10.1016/j.celrep.2017.12.069.
Berri, C., E. Le Bihan-Duval, M. Debut, V. Santé-Lhoutellier, E. Baéza, V. Gigaud, Y. Jégo, M. J. Duclos. 2007. Consequence of muscle hypertrophy on characteristics of Pectoralis major muscle and breast meat quality of broiler chickens. J. Anim. Sci. 85:2005–2011. https://doi.org/10.2527/jas.2006-398.
Blair, L. J., J. D. Baker, J. J. Sabbagh, and C. A. Dickey. 2015. The emerging role of peptidyl-prolyl isomerase chaperones in tau oligomerization, amyloid processing and Alzheimer’s disease. J. Neurochem. 133:1–13. https://doi.org/10.1111/jnc.13033.
Boonyarom, O., and K. Inui. 2006. Atrophy and hypertrophy of skeletal muscles: Structural and functional aspects. Acta Physiol. 188:77–89. https://doi.org/10.1111/j.1748-1716.2006.01613.x.
Bouter, A., C. Gounou, R. Bérat, S. Tan, B. Gallois, T. Granier, B. L. D’Estaintot, E. Pöschl, B. Brachvogel, and A. R. Brisson. 2011. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nat. Commun. 2:1–9.
Byron, M. D., X. Zhang, M. E. Von Staden, T. R. Jarvis, C. A. Crist, W. Zhai, and M. W. Schilling. 2020. Impact of refridgerated storage time on the dissipation of woody broiler breast meat. Meat Muscle Biology. 4(1). https://doi.org/10.22175/mmb.9477.
Cai, K., W. Shao, X. Chen, Y. L. Campbell, M. N. Nair, S. P. Suman, C. M. Beach, M. C. Guyton, and M. W. Schilling. 2018. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poultry Sci. 97:337–346. https://doi.org/10.3382/ps/pex284.
Chen, S., H. Jiang, H. Peng, X. Wu, and J. Fang. 2017. The utility of ovotransferrin and ovotransferrin-derived peptides as possible candidates in the clinical treatment of cardiovascular diseases. Oxid. Med. Cell. Longev. 2017:1–6. https://doi.org/10.1155/2017/6504518.
Chen, F., Y. Sugiura, K. G. Myers, Y. Liu, and W. Lin. 2010. Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction. P. Natl. Acad. Sci. USA. 107:1636–1641. https://doi.org/10.1073/pnas.0911516107.
Christensen, M. V, C. K. Høgdall, K. M. J. Umsen, and E. V. S. Høgdall. 2018. Annexin A2 and cancer: A systematic review. Int. J. Oncol. 52:5–18. https://doi.org/10.3892/ijo.2017.4197.
Coghlin, C., B. Carpenter, S. R. Dundas, L. C. Lawrie, C. Telfer, and G. I. Murray. 2006. Characterization and over-expression of chaperonin t-complex proteins in colorectal cancer. J. Pathol. 210:351–357. https://doi.org/10.1002/path.2056.
Cooper, C. A., M. L. Tizard, T. Stanborough, S. C. Moore, P. S. Chandry, K. A. Jenkins, T. G. Wise, T. E. O’Neil, D. S. Layton, K. R. Morris, R. J. Moore, N. Fegan, and T. J. Doran. 2019. Overexpressing ovotransferrin and avian β-defensin-3 improves antimicrobial capacity of chickens and poultry products. Transgenic Res. 28:51–76. https://doi.org/10.1007/s11248-018-0101-2.
Cowling, B. S., M. J. McGrath, M. A. Nguyen, D. L. Cottle, A. J. Kee, S. Brown, J. Schessl, Y. Zou, J. Joya, C. G. Bönnemann, E. C. Hardeman, and C. A. Mitchell. 2008. Identification of FHL1 as a regulator of skeletal muscle mass: Implications for human myopathy. J. Cell Biol. 183:1033–1048. https://doi.org/10.1083/jcb.200804077.
Cui, X., Z. P. Hu, Z. Li, P. J. Gao, and J. Y. Zhu. 2015. Overexpression of chaperonin containing TCP1, subunit 3 predicts poor prognosis in hepatocellular carcinoma. World J. Gastroentero. 21:8588–8604. https://doi.org/10.3748/wjg.v21.i28.8588.
D’Alessandro, A., C. Marrocco, V. Zolla, M. D’Andrea, and L. Zolla. 2011. Meat quality of the longissimus lumborum muscle of Casertana and Large White pigs: Metabolomics and proteomics intertwined. J. Proteomics. 75:610–627. https://doi.org/10.1016/j.jprot.2011.08.024.
Demonbreun, A. R., K. S. Fallon, C. C. Oosterbaan, E. Bogdanovic, J. L. Warner, J. J. Sell, P. G. Page, M. Quattrocelli, D. Y. Barefield, and E. M. McNally. 2019. Recombinant annexin A6 promotes membrane repair and protects against muscle injury. J. Clin. Invest. https://doi.org/10.1172/JCI128840.
Desai, M. A., V. Jackson, W. Zhai, S. P. Suman, M. N. Nair, C. M. Beach, and M. W. Schilling. 2016. Proteome basis of pale, soft, and exudative-like (PSE-like) broiler breast (Pectoralis major) meat. Poultry Sci. 95:2696–2706. https://doi.org/10.3382/ps/pew213.
Desai, M. A., P. Joseph, S. P. Suman, J. L. Silva, T. Kim, and M. W. Schilling. 2014. Proteome basis of red color defect in channel catfish (Ictalurus punctatus) fillets. Lebensm.-Wiss. Technol. 57:141–148. https://doi.org/10.1016/j.lwt.2014.01.001.
Evans, T. W. 2002. Review article: Albumin as a drug--biological effects of albumin unrelated to oncotic pressure. Aliment. Pharm. Ther. 16:6–11. https://doi.org/10.1046/j.1365-2036.16.s5.2.x.
Franco, D., A. Mato, F. J. Salgado, M. Lopez-Pedrouso, M. Carrera, S. Bravo, M. Parrado, J. M. Gallardo, and C. Zapata. 2015. Tackling proteome changes in the longissimus thoracis bovine muscle in response to pre-slaughter stress. J. Proteomics. 122:73–85. https://doi.org/10.1016/j.jprot.2015.03.029.
Gao, H., S. Hartnett, and Y. Li. 2017. Ubiquitin C-Terminal Hydrolase L1 regulates myoblast proliferation and differentiation. Biochem. Bioph. Res. Co. 492:96–102. https://doi.org/10.1016/j.bbrc.2017.08.027.
Geers, C., and G. Gros. 2000. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol. Rev. 80:681–715. https://doi.org/10.1152/physrev.2000.80.2.681.
Gerke, V., and S. E. Moss. 2002. Annexins: From structure to function. Physiol. Rev. 82:331–371. https://doi.org/10.1152/physrev.00030.2001.
Harding, J. W., E. A. Pyeritz, E. S. Copeland, and B. White. 1975. Role of glycerol 3 phosphate dehydrogenase in glyceride metabolism. Effect of diet on enzyme activities in chicken liver. Biochem. J. 146:223–229. https://doi.org/10.1042/bj1460223.
Hartl, F. U., A. Bracher, and M. Hayer-Hartl. 2011. Molecular chaperones in protein folding and proteostasis. Nature. 475:324–332. https://doi.org/10.1038/nature10317.
Huang, X., and D. U. Ahn. 2018. The incidence of muscle abnormalities in broiler breast meat - A review. Korean J. Food Sci. An. 38:835–850. https://doi.org/10.5851/kosfa.2018.e2.
Hubert, S. M., T. J. Williams, and G. Athrey. 2018. Insights into the molecular basis of wooden breast based on comparative analysis of fast-and slow-growth broilers. June 27, 2018. bioRxiv. https://doi.org/10.1101/356683.
Joseph, P., S. P. Suman, G. Rentfrow, S. Li, and C. M. Beach. 2012. Proteomics of muscle-specific beef color stability. J. Agr. Food Chem. 60:3196–3203. https://doi.org/10.1021/jf204188v.
Kamelgarn, M., J. Chen, L. Kuang, H. Jin, E. J. Kasarskis, and H. Zhu. 2018. ALS mutations of FUS suppress protein translation and disrupt the regulation of nonsense-mediated decay. P. Natl. Acad. Sci. USA. 115:E11904–E11913. https://doi.org/10.1073/pnas.1810413115.
Kawasaki, T., T. Iwasaki, M. Yamada, T. Yoshida, and T. Watanabe. 2018. Rapid growth rate results in remarkably hardened breast in broilers during the middle stage of rearing: A biochemical and histopathological study. PLoS One. 13:e0193307. https://doi.org/10.1371/journal.pone.0193307.
Keller, A., J. Demeurie, T. Merkulova, G. Géraud, C. Cywiner-Golenzer, M. Lucas, and F. P. Châtelet. 2000. Fibre-type distribution and subcellular localisation of α and β enolase in mouse striated muscle. Biol. Cell. 92:527–535. https://doi.org/10.1016/s0248-4900(00)01103-5.
Kubota, H., K. Willison, A. Ashworth, M. Nozaki, H. Miyamoto, H. Yamamoto, A. Matsushiro, and T. Morita. 1992. Structure and expression of the gene encoding mouse t-complex polypeptide (Tcp-1). Gene. 120:207–215. https://doi.org/10.1016/0378-1119(92)90095-7.
Kuttappan, V. A., W. Bottje, R. Ramnathan, S. D. Hartson, C. N. Coon, B. W. Kong, C. M. Owens, M. Vazquez-A Non, and B. M. Hargis. 2017. Proteomic analysis reveals changes in carbohydrate and protein metabolism associated with broiler breast myopathy. Poultry Sci. 96:2992–2999. https://doi.org/10.3382/ps/pex069.
Kuttappan, V. A., B. M. Hargis, and C. M. Owens. 2016. White striping and woody breast myopathies in the modern poultry industry: a review. Poultry Sci. 95:2724–2733. https://doi.org/10.3382/ps/pew216.
Lana, A., and L. Zolla. 2016. Proteolysis in meat tenderization from the point of view of each single protein: A proteomic perspective. J. Proteomics. 147:85–97. https://doi.org/10.1016/j.jprot.2016.02.011.
Lee, S. P., C. M. Park, K. S. Kim, E. Kim, M. Jeong, J. Y. Shin, C. H. Yun, K. Kim, P. B. Chock, and H. Z. Chae. 2018. Structural and biochemical analyses reveal ubiquitin C-terminal hydrolase-L1 as a specific client of the peroxiredoxin II chaperone. Arch. Biochem. Biophys. 640:61–74. https://doi.org/10.1016/j.abb.2018.01.003.
Lefaucheur, L. 2010. A second look into fibre typing--Relation to meat quality. Meat Sci. 84:257–270. https://doi.org/10.1016/j.meatsci.2009.05.004.
Li, L. J., L. S. Zhang, Z. J. Han, Z. Y. He, H. Chen, and Y. M. Li. 2017. Chaperonin containing TCP-1 subunit 3 is critical for gastric cancer growth. Oncotarget. 8:111470–111481. https://doi.org/10.18632/oncotarget.22838.
Liu, H., J. Ding, F. Chen, B. Fan, N. Gao, Z. Yang, and L. Qi. 2010. Increased expression of elongation factor-1α is significantly correlated with poor prognosis of human prostate cancer. Scand. J. Urol. Nephrol. 44:277–283. https://doi.org/10.3109/00365599.2010.492787.
Liu, X., H. Qu, Y. Zheng, Q. Liao, L. Zhang, X. Liao, X. Xiong, Y. Wang, R. Zhang, H. Wang, Q. Tong, Z. Liu, H. Dong, G. Yang, Z. Zhu, J. Xu, and H. Zheng. 2018. Mitochondrial glycerol 3 phosphate dehydrogenase promotes skeletal muscle regeneration. EMBO Mol. Med. 10:e9390. https://doi.org/10.15252/emmm.201809390.
Livingston, M. L., P. R. Ferket, J. Brake, and K. A. Livingston. 2019. Dietary amino acids under hypoxic conditions exacerbates muscle myopathies including wooden breast and white stripping. Poultry Sci. 98:1517–1527. https://doi.org/10.3382/ps/pey463.
Malila, Y., K. Thanatsang, S. Arayamethakorn, T. Uengwetwanit, Y. Srimarut, M. Petracci, G. M. Strasburg, W. Rungrassamee, and W. Visessanguan. 2019. Absolute expressions of hypoxia-inducible factor-1 alpha (HIF1A) transcript and the associated genes in chicken skeletal muscle with white striping and wooden breast myopathies. PLoS One. 14:e0220904. https://doi.org/10.1371/journal.pone.0220904.
Mangaraj, M., and R. Nanda. 2016. Apolipoprotein A-I: A molecule of diverse function. Indian Journal of Clinical Biochemistry. 31:253–259. https://doi.org/10.1007/s12291-015-0513-1.
McGrath, M. J., D. L. Cottle, M. Nguyen, J. M. Dyson, I. D. Coghill, P. A. Robinson, M. Holdsworth, B. S. Cowling, E. C. Hardeman, C. A. Mitchell, and S. Brown. 2006. Four and a half LIM protein 1 binds myosin-binding protein C and regulates myosin filament formation and sarcomere assembly. J. Biol. Chem. 281:7666–7683. https://doi.org/10.1074/jbc.M512552200.
Merlot, A. M., D. S. Kalinowski, and D. R. Richardson. 2014. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 5:299. https://doi.org/10.3389/fphys.2014.00299.
Mounier, N., and A. P. Arrigo. 2002. Actin cytoskeleton and small heat shock proteins: How do they interact? Cell Stress Chaperon. 7:167–176. https://doi.org/10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2.
Munoz, L. E., S. Franz, F. Pausch, B. Fürnrohr, A. Sheriff, B. Vogt, P. M. Kern, W. Baum, C. Stach, D. Von Laer, B. Brachvogel, E. Poschl, M. Herrmann, and U. S. Gaipl. 2007. The influence on the immunomodulatory effects of dying and dead cells of Annexin V. J. Leukocyte Biol. 81:6–14. https://doi.org/10.1189/jlb.0306166.
Mutryn, M. F., E. M. Brannick, W. Fu, W. R. Lee, and B. Abasht. 2015. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genomics. 16:1–19.
Nakagawa, T., H. Zhu, N. Morishima, E. Li, J. Xu, B. A. Yankner, and J. Yuan. 2000. Caspase-12 mediates endoplasmic reticulum specific apoptosis and cytotoxicity by amyloid-b. Nature. 403:98–103. https://doi.org/10.1038/47513.
Owens, C. M. 2014. Identifying quality defects in poultry processing. Watt AgNet. 42–50. https://www.wattagnet.com/articles/22065-identifying-quality-defects-in-poultry-processing?v=preview. (Accessed 30 September 2019).
Papah, M.B., and B. Abasht. 2019. Dysregulation of lipid metabolism and appearance of slow myofiber-specific isoforms accompany the development of Wooden Breast myopathy in modern broiler chickens. Sci. Rep.-UK. 9:17170. https://doi.org/10.1038/s41598-019-53728-8.
Papalouka, V., D. A. Arvanitis, E. Vafiadaki, M. Mavroidis, S. A. Papadodima, C. A. Spiliopoulou, D. T. Kremastinos, E. G. Kranias, and D. Sanoudou. 2009. Muscle LIM protein interacts with cofilin 2 and regulates F-actin dynamics in cardiac and skeletal muscle. Mol. Cell. Biol. 29:6046–6058. https://doi.org/10.1128/MCB.00654-09.
Petracci, M., S. Mudalal, F. Soglia, and C. Cavani. 2015. Meat quality in fast-growing broiler chickens. World Poultry Sci. J. 71:363–374. https://doi.org/10.1017/S0043933915000367.
Petracci, M., F. Soglia, M. Madruga, L. Carvalho, E. Ida, and M. Estevez. 2019. Wooden breast, white striping, and spaghetti meat: Causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. F. 18:565–583. https://doi.org/10.1111/1541-4337.12431.
Reutelingsperger, C. P. M., J. M. M. Kop, G. Hornstra, and H. C. Hemker. 1988. Purification and characterization of a novel protein from bovine aorta that inhibits coagulation: Inhibition of the phospholipid dependent factor Xa catalyzed prothrombin activation, through a high affinity binding of the anticoagulant to the phospholipids. Eur. J. Biochem. 173:171–178. https://doi.org/10.1111/j.1432-1033.1988.tb13981.x.
Scheffler, T. L., and D. E. Gerrard. 2007. Mechanisms controlling pork quality development: The biochemistry controlling postmortem energy metabolism. Meat Sci. 77:7–16. https://doi.org/10.1016/j.meatsci.2007.04.024.
Sihvo, H. K., N. Airas, J. Lindén, and E. Puolanne. 2018. Pectoral vessel density and early ultrastructural changes in broiler chicken wooden breast myopathy. J. Comp. Pathol. 161:1–10. https://doi.org/10.1016/j.jcpa.2018.04.002.
Sihvo, H. K., K. Immonen, and E. Puolanne. 2014. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 51:619–623. https://doi.org/10.1177/0300985813497488.
Silva, L. H. P., R. T. S. Rodrigues, D. E. F. Assis, P. D. B. Benedeti, M. S. Duarte, and M. L. Chizzotti. 2019. Explaining meat quality of bulls and steers by differential proteome and phosphoproteome analysis of skeletal muscle. J. Proteomics. 199:51–66. https://doi.org/10.1016/j.jprot.2019.03.004.
Soglia, F., S. Mudalal, E. Babini, M. Di Nunzio, M. Mazzoni, F. Sirri, C. Cavani, and M. Petracci. 2016. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poultry Sci. 95:651–659. https://doi.org/10.3382/ps/pev353.
Talapatra, S., J. D. O. Wagner, and C. B. Thompson. 2002. Elongation factor-1 alpha is a selective regulator of growth factor withdrawal and ER stress-induced apoptosis. Cell Death Differ. 9:856–861. https://doi.org/10.1038/sj.cdd.4401078.
Tang, S., F. Tabet, B. J. Cochran, L. F. Cuesta Torres, B. J. Wu, P. J. Barter, and K. A. Rye. 2019. Apolipoprotein A-I enhances insulin-dependent and insulin-independent glucose uptake by skeletal muscle. Sci. Rep.-UK. 9:1350. https://doi.org/10.1038/s41598-018-38014-3.
Tangirala, R., K. Tsukamoto, S. H. Chun, D. Usher, E. Puré, and D. J. Rader. 1999. Rapid regression of atherosclerosis induced by liver-directed gene transfer of apoE in apoE-deficient mice. Arterioscl. Throm. Vas. 19:2162–2170. https://doi.org/10.1161/01.ATV.19.9.2162.
Tijare, V. V., F. L. Yang, V. A. Kuttappan, C. Z. Alvarado, C. N. Coon, and C. M. Owens. 2016. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poultry Sci. 95:2167–2173. https://doi.org/10.3382/ps/pew129.
Tomaszewska-Gras, J., F. J. G. Schreurs, and J. Kijowski. 2011. Post mortem development of meat quality as related to changes in cytoskeletal proteins of chicken muscles. Brit. Poultry Sci. 52:189–201. https://doi.org/10.1080/00071668.2011.561281.
Vera, M., B. Pani, L. A. Griffiths, C. Muchardt, C. M. Abbott, R. H. Singer, and E. Nudler. 2014. The translation elongation factor eEF1A1 couples transcription to translation during heat shock response. Elife. 3:e03164. https://doi.org/10.7554/eLife.03164.
Wang, Y., Y. Huang, J. Yang, F. Q. Zhou, L. Zhao, and H. Zhou. 2018. Pyruvate is a prospective alkalizer to correct hypoxic lactic acidosis. Military Medical Research. 5:13. https://doi.org/10.1186/s40779-018-0160-y.
Windpassinger, C., B. Schoser, V. Straub, S. Hochmeister, A. Noor, B. Lohberger, N. Farra, E. Petek, T. Schwarzbraun, L. Ofner, W. N. Löscher, K. Wagner, H. Lochmüller, J. B. Vincent, and S. Quasthoff. 2008. An X-linked myopathy with postural muscle atrophy and generalized hypertrophy, termed XMPMA, is caused by mutations in FHL1. Am. J. Hum. Genet. 82:88–99. https://doi.org/10.1016/j.ajhg.2007.09.004.
Zambonelli, P., M. Zappaterra, F. Soglia, M. Petracci, F. Sirri, C. Cavani, and R. Davoli. 2017. Detection of differentially expressed genes in broiler pectoralis major muscle affected by White Striping - Wooden Breast myopathies. Poultry Sci. 95:2771–2785. https://doi.org/10.3382/ps/pew268.
Zhai, W., E. D. Peebles, M. W. Schilling, and Y. Mercier. 2016. Effects of dietary lysine and methionine supplementation on Ross 708 male broilers from 21 to 42 d of age (I): Growth performance, meat yield, and cost effectiveness. J. Appl. Poultry Res. 25:197–211. https://doi.org/10.3382/japr/pfw004.
Zhang, X., J. D. Hendrix, M. D. Byron, S. Mukherjee, Y. L. Campbell, W. Zhai, and M. W. Schilling. 2018. Broiler genetic strain and diet on the incidence of woody breast meat. Proceedings from AMSA’s 71st Reciprocal Meat Conference, Kansas City, MO, 25 June 2018.