Introduction
In the last 15 years, several published meat science reviews have focused on different aspects of meat color research (Table 1). Although the objectives of these reports ranged from fundamental to applied, during the last 3 decades a bright cherry-red meat color remains a top sensory characteristic for consumers making meat purchasing decisions (Carpenter et al., 2001; Prill et al., 2019). Significant product development efforts also have occurred using plant-based pigments to mimic a bright-red color in raw meat analogs (Reiley, 2019), further reiterating the importance of traditionally acquired sensory perception related to the quality of meat. Although a bright-red color of meat is not a solid predictor of microbiological safety, failure to meet consumer expectations for safe and wholesome meat can lead to economic losses and generation of organic waste. Meat is nutrient dense and highly perishable. Hence, the meat industry is utilizing various packaging and processing strategies to extend the shelf life and colorlife of fresh meat during grading, fabrication, value addition, and retail sales.
Summary of meat color reviews in the past 15 years
| Title | Reference |
|---|---|
| Irradiation effects on meat color - a review | Brewer, 2004 |
| Current research in meat color | Mancini and Hunt, 2005 |
| Where is MAP going? A review and future potential of modified atmosphere packaging for meat | McMillin, 2008 |
| Myoglobin and lipid oxidation interactions: Mechanistic bases and control | Faustman et al., 2010 |
| Pale soft exudative (PSE) and dark firm dry (DFD) meats: Causes and measures to reduce these incidences - a mini review | Adzitey and Nurul, 2011 |
| Myoglobin chemistry and meat color | Suman and Joseph, 2013 |
| A structural approach to understanding the interactions between color, water-holding capacity and tenderness | Hughes et al., 2014 |
| Improving beef color stability: Practical strategies and underlying mechanisms | Suman et al., 2014 |
| Application of proteomics to characterize and improve color and oxidative stability of muscle foods | Joseph et al., 2015 |
| Factors influencing internal color of cooked meats | Suman et al., 2016 |
| Advancements in meat packaging | McMillin, 2017 |
| Causes and contributing factors to “dark cutting” meat: current trends and future directions: A review | Ponnampalam et al., 2017 |
| Proteomics of color in fresh muscle foods | Nair et al., 2017a |
| The application of carbon monoxide in meat packaging needs to be re-evaluated within the EU: An overview | Van Rooyen et al., 2017 |
| Meat packaging solutions to current industry challenges: A review | Holman et al., 2018b |
| Exogenous and endogenous factors influencing color of fresh meat from ungulates | Neethling et al., 2017 |
| Role of mitochondria in beef color: A review | Ramanathan et al., 2018 |
| Mitochondrial functionality and beef color: A review of recent research | Ramanathan et al., 2019b |
| Meat color is determined not only by chromatic heme pigments but also, by the physical structure and achromatic light scattering properties of the muscle | Hughes et al., 2019 |
| Effect of high-pressure treatment on the color of fresh and processed meats: A review | Bak et al., 2019 |
| Biomolecular interactions governing fresh meat color in post-mortem skeletal muscle: A review | Ramanathan et al., 2020 |
| Variations in meat color due to factors other than myoglobin chemistry; a synthesis of recent findings | Purslow et al., 2020 |
Even though the fundamental factors that cause discoloration have not changed in the past several decades, modifications in management/feeding practices have improved carcass quality. Grass-finished cattle contribute only 3% of the total beef produced in the United States (Cheung et al., 2017), but in Europe, Brazil, Argentina, Uruguay, and Australia, grass finishing is the most common feeding practice. The type of feed can affect total energy intake, deposition of glycogen, and antioxidants (Apaoblaza et al., 2020), which in turn can influence meat color. Cattle genetics, selection, and management practices result in heavier carcasses, which has negative effects on rates of chill and pH decline. However, these negative effects have been partially offset by cattle having greater amounts of marbling commonly associated with better eating quality. Another major change that occurred during the last 15 years is the use of “omics” technology to enhance our knowledge related to protein and metabolite changes in postmortem muscles. This review will focus on applied aspects of deviations of meat color with particular emphasis on pH-dependent effects on muscle biochemistry.
Effect of pH on Muscle Structure and Meat Color
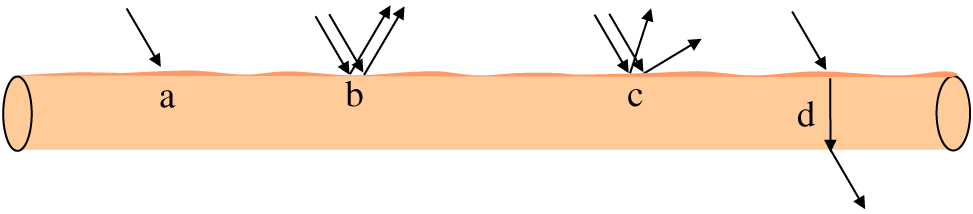
Postmortem muscle has a unique matrix that allows light to be absorbed, scattered, reflected, and transmitted (Figure 1). Previous studies (Swatland, 2003, 2008) and recent reviews (Hughes et al., 2017, 2019; Purslow et al., 2020) have discussed the impact of muscle ultrastructure on scattering of light and pigment absorbance at specific wavelengths. For example, the presence of hemeproteins such as myoglobin, hemoglobin, and cytochromes allow meat to absorb light in the Soret region (400–440 nm) and reflect light, especially in the red region (635–700 nm).
Schematic representation of light properties when light passes through a muscle fiber. (a) Absorption (light is absorbed by various components in meat, including heme proteins). (b) Reflectance (incident light is redirected; angle of incidence = angle of reflection; occurs at smooth surface). (c) Scattering (also called as diffuse reflection; incident light is diffused/reflected in different direction; occurs at irregular cut surface). (d) Refraction (bending of light waves when light passes from one medium to another). Adapted from Purslow et al., 2020; Swatland, 1989.
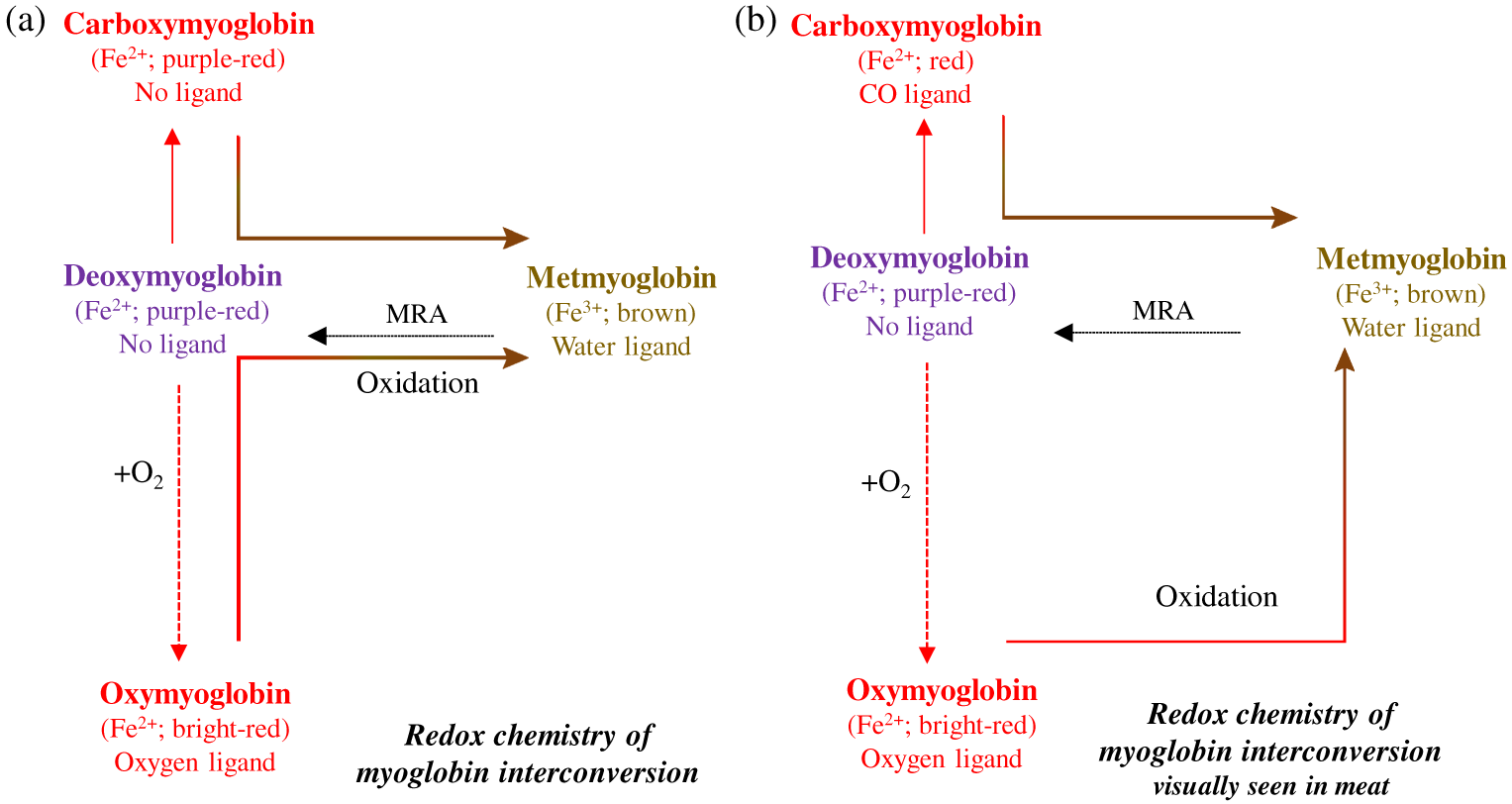
Myoglobin is the primary protein responsible for meat color, but hemoglobin and cytochromes also contribute to a lesser extent. Depending on the redox forms on fresh meat surfaces and the ligand bound, myoglobin can exist as deoxymyoglobin, oxymyoglobin, metmyoglobin, and carboxymyoglobin (Figure 2). Details of the interconversion of myoglobin redox forms and the role of oxygen partial pressure in redox chemistry are described in the American Meat Science Association Color Guide (AMSA, 2012). Each redox form can impart unique absorbance and reflectance spectra and their associated peaks/wavelength maxima (Table 2). Thus, in addition to muscle’s structural network, myoglobin forms also can influence the intensity of redness (saturation) of the meat.
Interconversion of different forms of myoglobin. (a) Oxymyoglobin is converted to metmyoglobin via deoxymyoglobin. (b) However, in fresh meat, visually one can observe conversion of bright-red color to metmyoglobin (without seeing a purple deoxymyoglobin form;. Adapted from AMSA, 2012. MRA = metmyoglobin reducing activity
Characteristics of each myoglobin form1 and impact on reflectance and absorbance
| Myoglobin forms | Soret peak2 (nm) | Absorption maxima (nm)2 | Reflectance maxima (nm) | Reflectance (%)3 | Absorbance4 |
|---|---|---|---|---|---|
| Deoxymyoglobin | 430 | 503 | 474 | 12.0 | 1.07 |
| Oxymyoglobin | 420 | 582 | 610 | 15.9 | 0.95 |
| Metmyoglobin | 410 | 503 | 572 | 14.7 | 1.04 |
| Carboxymyoglobin | 420 | 543 | 610 | 16.1 | 1.07 |
100% myoglobin standard were prepared using 2.54-cm-thick beef longissimus lumborum steaks according to AMSA (2012), and total reflectance and absorbance were calculated (Ramanathan et al., 2012).
Soret peak and absorption maxima recorded on steak (note: these values change if determined using purified myoglobin).
Reflectance = [ΣR400 to 700 nm ÷ 30]; represents amount of light reflected from beef steak surface.
Absorbance = [ΣA400 to 700 nm ÷ 30]; represents amount of light absorbed on beef steak surface.
The significance of heme proteins in meat color was highlighted by the removal of water-soluble chromophores by repeated washing (Swatland, 1989). The removal of chromophores increased reflectance due to the lack of molecules to absorb light. Although various factors can influence the optical properties of meat, the rate of pH decline postmortem, the ultimate postmortem muscle pH, and postharvest processes (such as product enhancement and packaging) play a significant role in muscle structural properties.
The homeostatic mechanisms of live muscles are severely challenged after exsanguination resulting in the stoppage of blood flow during animal harvest, which leads to the depletion of oxygen and a shift in muscle metabolism from aerobic to anaerobic (Donaldson and Lamont, 2013). Hence, glycolytic metabolism predominates, resulting in acidification of postmortem muscle. The drop in pH from physiological 7.2 to 5.6, and the rate of decline, have a profound effect on meat color and other physical traits of meat. Any alteration in the rate of decline or the ultimate postmortem muscle pH can influence both biochemical activity and muscle structure, which affects the formation of consumer-preferred bright-red color (English et al., 2016a). More specifically, a bright-red color in postmortem meat depends on myoglobin oxygenation, oxygen penetration, heme iron-reducing capabilities, the amount of free water on the surface of the meat, and the complex effects of the microstructure of the meat.
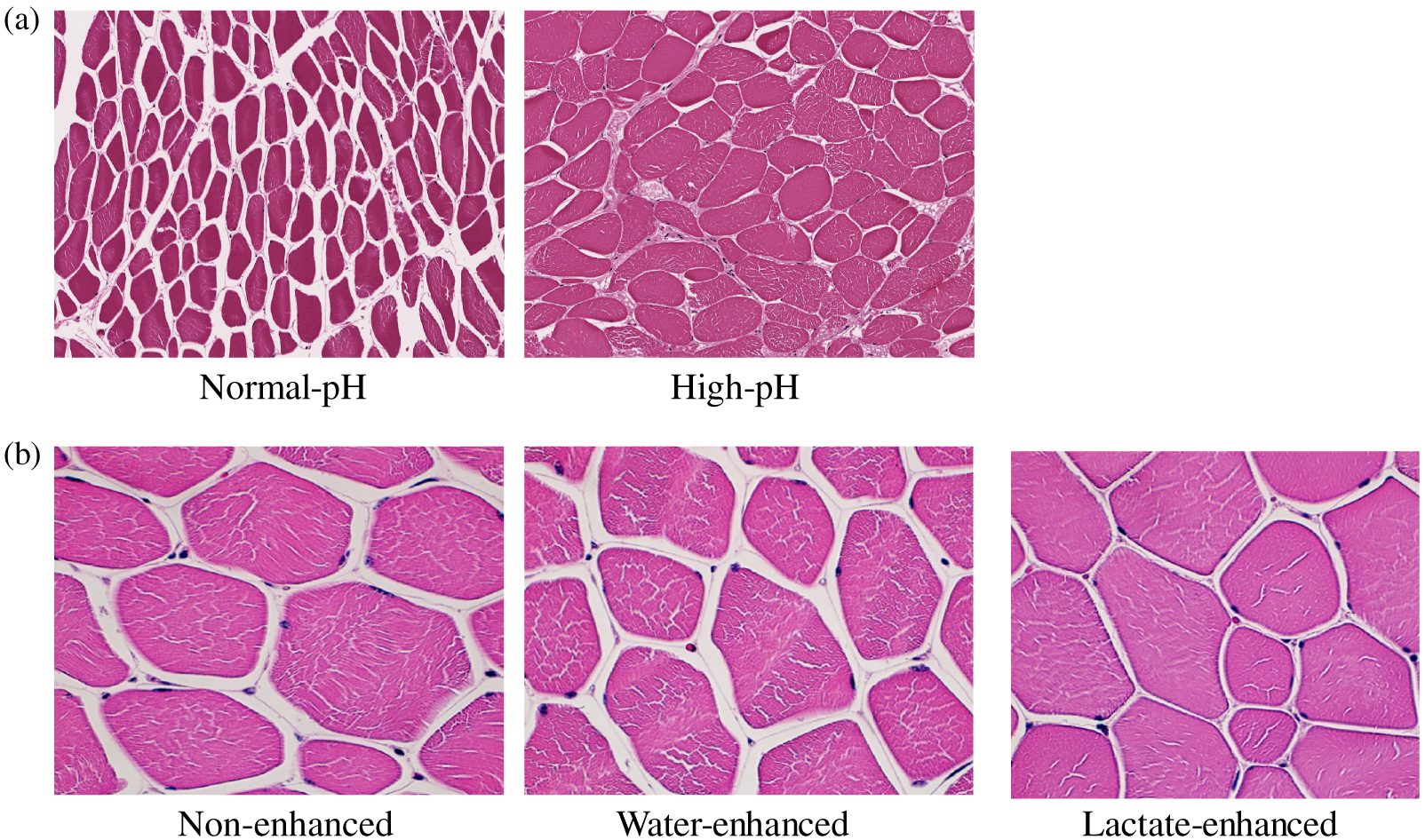
Dark-cutting and lactate-enhanced beef present 2 contrasting scenarios in which meat fails to have a bright-red color. In dark-cutting beef, the current knowledge indicates that preharvest chronic stress leads to mobilization of glycogen, which lessens the amount of muscle glycogen at death (McKeith et al., 2016; Mahmood et al., 2017), leading to greater-than-normal meat pH (classically, a pH ≥ 6.2, although some countries consider a pH ≥ 5.75 as darker-than-desired color). In contrast, normal-pH meat that is enhanced only with lactate (with no additional ingredients in the enhancement solution) darkens the bright-red color of meat by slightly elevating the normal pH due to greater enzymatic competition for oxygen (Ramanathan et al., 2009). A greater-than-normal muscle pH increases the negative charge on the muscle matrix and increases spaces within and between muscle myofibrils resulting in swelling and greater ability to hold more intracellular water, all of which decreases inter-fiber spaces and surface-free water, increases absorption of light, and decreases reflectance (Hughes et al., 2014a) as depicted in Figure 3a.
Effect of pH (a) and lactate-enhancement (b) on muscle structure. (a) Hematoxylin and eosin–stained section of normal-pH (pH = 5.6) and high-pH (pH 6.4) beef longissimus lumborum sections. Normal-pH sections have more interfiber space, while greater water-holding capacity of high-pH muscle leads to less interfiber space. Interfiber space was measured using ImageJ software. Magnification = 20×. (b) Hematoxylin and eosin–stained section of non-enhanced (pH = 5.6), water-enhanced (10% of green weight of muscles; pH 5.6), and potassium lactate (3% lactate in the final product; pH = 5.8)–enhanced beef longissimus lumborum muscles. Both non-enhanced and water-enhanced sections have greater interfiber space than lactate-enhanced sections. Interfiber space was measured using ImageJ software. Magnification = 40× (Ramanathan, Mancini, and Mafi, unpublished data, 2020).
Research determining the mechanistic basis of lactate-induced darkening of meat color (with no additional ingredients in the enhancement solution) noted that lactate alone could increase pH slightly (0.2 units vs. 0.6 in dark cutter), leading to muscle swelling and less reflectance (Figure 3b). Both dark cutting and lactate enhancement result in more tightly packed muscle bundles, leading to less water on the meat surface and less reflectance. In total, these muscle structural changes also increased the length of the light path through muscles, resulting in more absorption and less scattering (Swatland, 2008). Lactate enhancement can also increase the refractive index of the sarcoplasm (Brennecke et al., 2017). However, there were no differences in the refractive index properties of normal-pH versus dark-cutting sarcoplasmic extracts (Ramanathan, Mancini, and Mafi, unpublished data, 2020).
Discoloration in Fresh Meat
Several excellent reviews have detailed numerous aspects of myoglobin structure and biochemical mechanisms involving the dynamic blending of myoglobin redox forms in meat, especially metmyoglobin formation (Table 1). This review will focus on the mechanisms that influence fresh meat color, such as the competition for oxygen between myoglobin and mitochondria and oxygen-consuming enzymes and the reducing mechanism for metmyoglobin, all of which are intertwined with environmental factors of meat pH, oxygen partial pressures, meat temperature, muscles, and aging. Some of the data will apply directly to “the initial color developed,” whereas other data will apply to the “color stability” of the initial color. Regardless of this partitioning of terminology, the fundamental factors affecting fresh meat color are complex and highly interrelated, with factors ranging from live animal production to preharvest environmental issues to factors affecting the conversion of muscle to meat and numerous factors along the merchandising chain of meat marketing to consumers.
Oxygen available from the atmosphere or oxygen present within a package or processing environment are the main sources of oxygen for myoglobin and mitochondrial activity. Hence, there is competition for the available oxygen in postmortem muscle. Mitochondria and oxygen-consuming enzymes are the main competitors for the available oxygen (Tang et al., 2005). However, the competition for oxygen by mitochondria depends on the postmortem time and pH. For example, immediately after slaughter or if the pH of postrigor muscle is greater than normal, mitochondria are very active, which results in predominant deoxymyoglobin. Conversely, when mitochondria become less active during postmortem aging, there is limited oxygen consumption oxygen consumption by mitochondria. A practical implication is faster and greater blooming of aged steaks. But discoloration is often faster for steaks with greater storage times or with the addition of mitochondrial inhibitors (Cornforth and Egbert, 1985). In practical terms, these enzymes often play a role as an oxygen scavenger, which can have positive or negative effects depending on the desired color of meat.
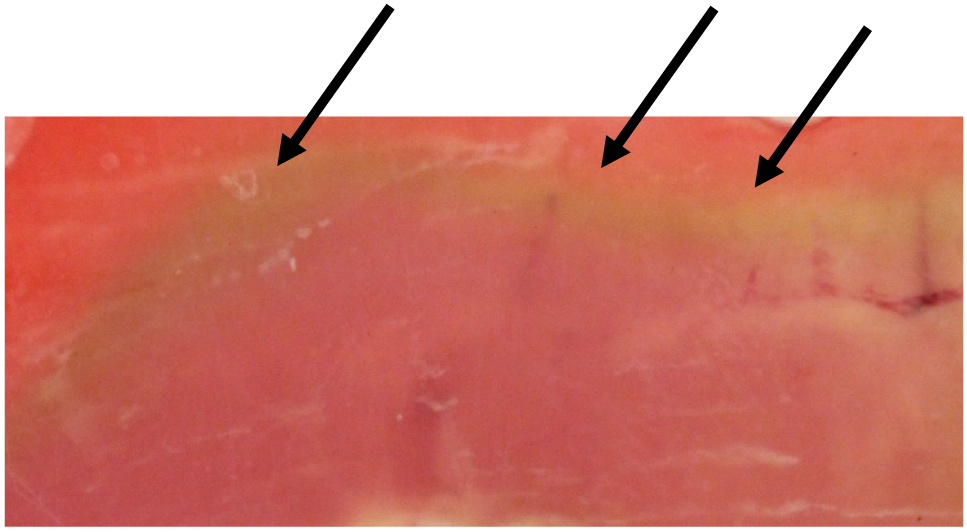
The redox state of iron and the type of ligand attached to the heme ring determines meat color. Oxygen partial pressures ≥ 159.2 mm Hg (atmospheric oxygen is 20.9%) provide a favorable condition for the formation of the bright-red color seen in polyvinyl chloride (PVC) or high-oxygen packages (50%–80% oxygen). For deoxymyoglobin to form in anaerobic packaging systems, nearly complete removal of oxygen from a package is required. As shown in Figure 4, a layer of metmyoglobin forms below the surface of the meat.
Migration of metmyoglobin layer from interior to surface. Beef longissimus lumborum sections (each 0.5 cm in thickness; aged >14d; pH 5.6) were placed between 2 glass slides (to create anaerobic conditions next to the glass), wrapped with aerobic film (to avoid drying and to promote oxygenation on the edge), and stored in the dark at 4°C for 2 d. Arrows indicate the metmyoglobin layer beneath the red surface (Ramanathan, Denzer, Mancini, and Mafi, unpublished data, 2020).
Myoglobin is unique in that it is prone to oxidation at low-oxygen partial pressures of 1–1.4 mm Hg in purified solution (George and Stratmann, 1952) and 6–7 mm Hg in intact meat (Ledward, 1970). Depending upon the surface oxygen concentration, the muscle structure, and the activity of mitochondria, oxygen will penetrate inside the meat until conditions favor auto-oxidation of both oxymyoglobin and deoxymyoglobin (Ledward, 1970). The thickness of the metmyoglobin layer begins at an oxygen concentration of about 3% oxygen (ca. partial pressure 23–24 mm Hg), at which point oxymyoglobin interfaces with metmyoglobin. The lower border of metmyoglobin ends at the border with deoxymyoglobin (≤1% oxygen and a partial pressure of oxygen of 7 mm Hg down to 1.5 mm Hg). The metmyoglobin layer will gradually move upward as the oxymyoglobin layer loses its ability to keep metmyoglobin reduced, resulting in discoloration and limiting meat color stability.
At the oxygen concentrations (1% to 3%) and their corresponding low partial pressures of oxygen (7.96 to 23.9 mm Hg) mentioned earlier, a large quantity of oxygen (31,000 to 10,450 parts per million [ppm]) still exists in the meat (1.04% oxygen concentration in package = 10,400 ppm of oxygen = 7.96 mm Hg of partial pressure of oxygen in package; AMSA, 2012), until two things happen. Additional oxygen must be removed, and the ferric heme iron must be reduced by the metmyoglobin reducing systems to assure that metmyoglobin will not lead to discoloration. If the meat temperature is too cold (<2°C), neither of these reactions will operate efficiency, thus the concept of “warm-phase management” (2–4°C for a short time) must be practiced to get the metmyoglobin reduced and the remaining oxygen reduced to essentially zero. It is well known that some muscles and ground products do not have the ability to remove sufficient oxygen and to also reduce the metmyoglobin in normal processing environments to form deoxymyoglobin. At initial vacuum levels ≤90%, metmyoglobin often is a problem (AMSA, 2012). Thus, in-package vacuum level for muscles of a normal pH that are classified as average or less-than-average in color stability is a critical parameter to monitor and control for many operations. (McKenna et al., 2005; Mohan et al., 2010b; Raines et al., 2010; Joseph et al., 2012; Ke et al., 2017).
If a bright-red surface color is desired, it is critical to minimize metmyoglobin formation on the surface after slicing. If sliced products are stored at 4°C–8°C and stacked (even for a short period), the freshly cut surfaces directly on other cut surfaces will form metmyoglobin. To minimize this discoloration, enhance the extent of bloom by having the meat surface at 0°C–2ºC to slow the oxygen-consuming competitors to allow the oxygen to penetrate as far as possible. Alternatively, the meat can be packaged in a modified atmosphere of 40%–80% oxygen, in which the metmyoglobin layer will form substantially farther below the surface.
Case-ready meat systems have allowed meat purveyors to process and package meat in a centralized location, which helps improve product consistency, efficiency, and cost reduction. Case-ready is good for atmospheric or high-oxygen packaging systems with strict temperature control (McMillin, 2008). This packaging works well using a “mother bag” containing multiple packages at the desired oxygen level. Case-ready also can be done with anaerobic packaging; however, both cold-chain plus warm-chain management should be considered to eliminate discoloration. If done properly, the carbon monoxide (CO) system is great and has all the advantages of an anaerobic system but with meat being bright red. The use of the “mother bag” concept also works with anaerobic packaging. With recent consumer interest to provide juicier and more flavorful hamburger patties, the industry started shipping fresh never-frozen ground beef patties to stores. Although packaging patties in vacuum is ideal, due to potential deformity, purveyors often prefer to ship fresh patties in anaerobic mother bags.
Enhancing meat with lactate in case-ready meat has received attention, and several studies have determined its color-stabilizing effect in beef (Kim et al., 2006; Mancini et al., 2009). Although lactate is primarily used as an antimicrobial, lactate’s role in stabilizing color can be attributed to its ability to regenerate nicotinamide adenine dinucleotide reduced form (NADH) via lactate dehydrogenase activity. In addition to physical changes induced by lactate enhancement, biochemical mechanisms also contribute to muscle darkening. In dark-cutting beef, a greater than normal pH promotes mitochondrial activity and oxygen consumption, leading to predominant deoxymyoglobin and darker meat color (English et al., 2016b). Conversely, lactate-induced darkening occurs when normal-pH steaks are enhanced with lactate. The excessive addition of lactate reversed the normal direction of lactate dehydrogenase activity, resulting in increased NADH and pyruvate and less metmyoglobin (Kim et al., 2006). Details of these reactions were studied by Ramanathan et al. (2013), who reported that deoxymyoglobin content increased when mitochondria were incubated with lactate and oxymyoglobin. The role of mitochondria in lactate-induced darkening was validated by the addition of rotenone (complex I mitochondrial inhibitor). Incubating muscle homogenates with lactate and rotenone reversed lactate-induced muscle darkening (Ramanathan et al., 2009). In summary, alteration in ultimate muscle pH can influence muscle ultrastructure and biochemistry, which can affect optical properties and associated sensory perception. In addition to enhancement technology, case-ready packaging also allows the adding of antioxidants, using active packaging films, and using processes such as high-pressure processing to improve safety and limit meat quality deterioration (Table 3).
Effects of various postharvest processes on meat color
| Category | Impact | Reference |
|---|---|---|
| Packaging | Vacuum skin packaging increased the color stability of fresh beef | Nassu et al. (2012) |
| Active antioxidant films | Rosemary active packaging enhanced stability of fresh beef color in aerobic conditions Eugenol film in direct contact with ground beef improved color stability |
Sirocchi et al. (2017) Park et al. (2012) |
| Red color of ground beef patties stabilized with 0.5% and 1.0% citric acid in the packaging film | Battisti et al. (2017) | |
| Starch films in combination with green tea and butylated hydroxyltolune retarded metmyoglobin formation in beef | Nisa et al. (2015) | |
| Oregano-containing films extended shelf life of beef and lamb color | Camo et al. (2008); Zinoviadou et al. (2009); Djenane et al. (2016) | |
| Combination of rosemary film and HiOx-MAP improved beef color and lamb color stability | Nerín et al. (2006); Sirocchi et al. (2017) | |
| Active film containing citric acid was redder than control packaging | Júnior et al. (2015) | |
| Nitrite-embedded packaging | Nitrite film improved red color of beef steaks, ground beef patties, pork chops, and pork sausage | Yang et al. (2016a, 2016b) |
| Red color improvement occurred 5 d after packaging of beef steaks in nitrite-embedded packaging, repackaging of nitrite film beef steaks in PVC significantly decreased a* values in beef steaks in 24 h | Claus and Du (2013) | |
| Nitrite film improved red color of dark cutting steaks in 24 h | Ramanathan et al. (2018) | |
| Modified atmospheric packaging | Compared with PVC overwrap, red color of lamb had higher stability in HiOx-MAP | Warner et al. (2017) |
| Lean color improvement and stability in CO-MAP and HiOx-MAP was muscle dependent | John et al. (2005); Mancini et al. (2009); Kim et al. (2010) | |
| HiOx-MAP increased surface redness of beef | O’Grady et al. (2000); John et al. (2005); Kim et al. (2010); Mancini et al. (2010); Suman et al. (2010b); Mancini et al. (2011); Resconi et al. (2012) | |
| Red color and color stability were enhanced through CO-MAP of beef | John et al. (2005); Brooks et al. (2008); Grobbel et al. (2008a); Grobbel et al. (2008b); Jeong and Claus (2010); Mancini et al. (2010) | |
| Increased a* values of beef in HiOx-MAP systems with 50% and 80% oxygen | Esmer et al. (2011) | |
| Pork color improved in 1% CO-MAP system | Viana et al. (2005) | |
| Repackaging of CO-MAP steaks decreased the color life of steaks and ground beef | Hunt et al. (2004); Jeong and Claus (2010) | |
| Combination of 1% chitosan and CO-MAP increased redness of ground beef | Suman et al. (2010b) | |
| CO-MAP and HiOx-MAP improved red color of dark-cutting beef | Wills et al. (2017); Zhang et al. (2018) | |
| HiOx packaging improved color stability of ground beef patties enhanced with pyruvate compared with PVC packaging | Ramanathan et al. (2012) | |
| Oxygen scavengers | Ground beef discoloration was decreased in the presence of FreshPax (Multisorb Filtration Group, Buffalo, NY) oxygen scavengers | Gill and McGinnis (1995) |
| Antioxidants | Chitosan improved redness and decreased discoloration of ground beef patties | Georgantelis et al. (2007); Suman et al. (2010b) |
| Butterbur and broccoli extracts extended color stability of ground beef | Kim et al. (2013b) | |
| Chamnamul and fatsia decreased a* values of ground beef patties | Kim et al. (2013a) | |
| Eugenol inhibited metmyoglobin formation and improved color stability of ground beef | Allen and Cornforth (2010) | |
| Noni puree increased red color stability in beef patties | Tapp et al. (2012) | |
| Olive oil extract and lutenin improved shelf life of beef patties | Hayes et al. (2010) | |
| Gallic red wine, prune, and grape seed extract increased storage life of ground beef patties compared with control | Bouarab-Chibane et al. (2017) | |
| Black currant extract stabilized red color and darkened the color of raw pork patties during storage | Jia et al. (2012) | |
| Mushroom extract extended shelf life by retarding metmyoglobin formation | Bao et al. (2008) | |
| Ground beef patties with plum and black rice extract decreased L* values and increased a* values compared with control patties | Yıldız-Turp and Serdaroglu (2010); Prommachart et al. (2020) | |
| Fenugreek seeds and ginger improved color stability of beef patties | Mansour and Khalil (2000) | |
| Grape seed extract increased a* values in fresh pork patties | Carpenter et al. (2007) | |
| Color changes under aerobic storage conditions were minimal in pork patties containing grape pomace extract | Garrido et al. (2011) | |
| Rosemary | Addition of rosemary extract to beef and lamb increased color stability | Balentine et al. (2006); Allen and Cornforth (2010) |
| Dark-cutting beef enhanced with rosemary had greater redness than nonenhanced dark-cutting beef | Wills et al. (2017) | |
| Rosemary improved color stability of beef and lamb packaged in HiOx-MAP | Sánchez-Escalante et al. (2001); Sánchez-Escalante et al. (2003); Brooks et al. (2008); Camo et al. (2008) | |
| In combination with rosemary, chitosan, and tocopherol increased shelf life of beef patties compared with control patties | Georgantelis et al. (2007) | |
| Ascorbic acid | Color stability of ground beef increased with ascorbic acid + rosemary in HiOx-MAP packaging | Sánchez-Escalante et al. (2001); Djenane et al. (2002); Sánchez-Escalante et al. (2003); Sánchez-Escalante et al. (2011) |
| Carnosine + ascorbic acid in HiOx-MAP packaging increased color stability | Djenane et al. (2004) | |
| Vitamin C enhancement can increase beef’s retail life | Wheeler et al. (1996) | |
| Ascorbic acid in combination with carnosine or taurine improved red color and increased shelf life of fresh beef patties in HiOx-MAP | Sánchez-Escalante et al. (2011) | |
| Ground beef patties maintained red color with the addition of ascorbic acid and α-tocopherol compared with control patties | Ismail et al. (2008) | |
| Enhancements | Acetic acid improved red color of high-pH beef steaks | Tapp et al. (2017) |
| Succinate addition decrease L* values but increased metmyoglobin reduction in ground beef | Mancini et al. (2011) | |
| Ground beef containing pyruvate had extended shelf life in HiOx-MAP and PVC compared with control ground beef | Ramanathan et al. (2012) | |
| Citric acid enhancement had limited effects on dark-cutting beef fresh color | Stackhouse et al. (2016) | |
| Lactate | Lactate improved color stability and increased a* values of beef in HiOx-MAP | Kim et al. (2009); Mancini et al. (2009); Mancini et al. (2010); Suman et al. (2010a) |
| Lactate enhancement darkened beef color | Kim et al. (2006); Knock et al. (2006); Grobbel et al. (2008a); Ramanathan et al. (2010) | |
| Lactate-enhancement stabilized surface color of beef steaks | Kim et al. (2006); Knock et al. (2006) | |
| Addition of pyruvate, succinate, and lactate decreased discoloration, increased redness, and darkened beef steaks; pyruvate enhancement resulted in most surface darkening | Ramanathan et al. (2011) | |
| Improving redness in the presence of pyruvate, malate, and lactate was muscle-dependent | Mohan et al. (2010b) | |
| Fresh color of dark-cutting beef improved with lactic acid enhancement | Sawyer et al. (2009); Sawyer et al. (2011); Sharma et al. (2014) | |
| High-pressure processing | High-pressure processing promotes discoloration, and meat becomes pale in appearance | Bak et al. (2019) |
a* = redness; L* = lightness; CO-MAP = carbon monoxide modified atmospheric packaging; HiOx-MAP = high-oxygen modified atmospheric packaging; PVC = polyvinyl chloride.
Effects of Extended Aging on Color Stability
Postmortem muscle is not biochemically inert, as numerous studies (Bendall and Taylor, 1972; Ledward, 1972; Tang et al., 2005; Seyfert et al., 2006; Ramanathan et al., 2013; Mancini and Ramanathan, 2014; English et al., 2016a; Nair et al., 2018) have shown that the metabolic mechanisms (both enzymatic and nonenzymatic) remain functional when the required metabolites, temperature, or/and pH conditions are favorable, but the chemistry can either enhance or hinder meat color as shown in Figure 2. These reactions vary by muscle and become less effective as the postmortem age of meat increases (AMSA, 2012).
Wet aging in vacuum packages is a common method to extend shelflife, enhance eating quality, and preserve meat (Kim et al., 2017). The 2015 US National Beef Tenderness Survey reported that beef sold at retail had post-fabrication aging time ranging from 6 to 102 d (Guelker et al., 2013). Extended aging can also be helpful in the export market. However, an extended aging period has a detrimental effect on color stability (English et al., 2016a). Steaks aged more than 14 d discolor faster during retail display. The research determining the fundamental basis of lower color stability in aged meat indicated that metmyoglobin reducing activity, oxygen consumption of steaks, mitochondrial activity, and mitochondrial content decreased with the aging period. Since metabolites and enzymes contribute a major role in metmyoglobin reducing activity, Ma et al. (2017) reported that NADH and glutathione content in beef muscles decreased with increasing aging time. Metabolites such as fumaric acid, creatinine, and fructose, which take part in the glycolytic/tricarboxylic acid cycle and can regenerate NADH, decreased with aging and display time (Mitacek et al., 2019). Although NADH concentration decreased during aging, there was no difference in NADH-dependent reductase activity during 28-d aging (Mitacek et al., 2019). However, muscles and aging times that result in improved color stability are often associated with greater glycolytic enzymes activity.
Increased oxygen consumption can be detrimental to the formation of bright-red color. For example, extended aging time can decrease mitochondrial activity, and one can assume steaks will bloom better due to less mitochondrial competition for oxygen. However, aging past 42 d tends to limit bloom. More specifically, steaks aged for 60 d had lower bloom than steaks aged 21 d (English et al., 2016a). This decreased bloom seems to be associated with less myoglobin available to oxygenate due to greater pigment lost as purge in postmortem aged meat.
Extended aged steaks packaged in high-oxygen modified atmospheric packaging (HiOx-MAP) had lower color stability compared with PVC packaging. A greater aging period can decrease antioxidant capacity, NADH level, and mitochondrial content (Mitacek et al., 2019). Hence, more oxidative conditions, as in HiOx-MAP, may have decreased color stability. However, packaging steaks in carbon monoxide modified atmospheric packaging (CO-MAP) retained red color throughout the display (English et al., 2016a; Ramanathan et al., 2019a). This suggests that CO-MAP may be an option if extended aged steaks are utilized for retail display. As aging time increased, metmyoglobin reducing activity and oxygen consumption decreased for normal- and high-pH steaks when packaged in PVC and HiOx-MAP compared with CO-MAP. In summary, extended aging is detrimental to color stability; however, the use of CO-MAP or the use of antioxidants in combination with packaging has the potential to improve the meat color of extended aged meat.
Adapting Packaging to Complement the Needs of High-pH Meat
Meat packaging serves to extend shelflife (microbial driven) and colorlife (visually driven), offers an opportunity for the consumer to make assumptions about quality, offers convenience in shipping, and limits meat waste. McMillian (2017) discussed in detail the newer packaging systems used in the meat industry. Aerobic (presence of oxygen) and anaerobic (no oxygen) packaging are the 2 major classifications of meat packaging. Aerobic packaging such as PVC overwrap or HiOx-MAP uses oxygen to provide an attractive bright-red color, whereas anaerobic packaging includes vacuum or no–oxygen-modified packaging flushed with CO or nitrogen. MAP represents rigid trays with oxygen-impermeable films, which helps to modify the gas compositions within a package. In HiOx-MAP, 80% oxygen is flushed (4 times the oxygen content of PVC), whereas in anaerobic MAP, gases such as CO, nitrogen, or carbon dioxide are flushed inside a package.
Greater oxygen content increases lipid oxidation, which can produce reactive primary and secondary lipid oxidation products (Faustman et al., 2010). Various studies have shown that lipid oxidation products can lower the activity of enzymes involved in metmyoglobin reducing activity and oxygen consumption (Liu et al., 2014; Zhai et al., 2019). Furthermore, 4-hydroxy-2-nonenal (HNE; a secondary lipid oxidation product) can bind with oxymyoglobin and promote oxidation (Alderton et al., 2003; Suman et al., 2007). Meat packaged in HiOx-MAP had lower metmyoglobin reducing activity and oxygen consumption compared with vacuum/PVC/CO-MAP (Seyfert et al., 2007; English et al., 2016a). Research determining the mechanistic basis of lower metmyoglobin reducing activity and oxygen consumption indicated that lipid oxidation products can decrease the activity of lactic dehydrogenase and NADH-dependent reductase (2 enzymes critical for meat color stability; Ramanathan et al., 2014; Zhai et al., 2019). Furthermore, NADH content was lower in HiOx-MAP compared with CO-MAP (Liu et al., 2014). Proteomic analysis demonstrated that glycolytic and energy metabolic enzymes important in NADH regeneration and antioxidant processes were more abundant in CO-MAP steaks than HiOx-MAP (Yang et al., 2018). In addition, glutathione was greater in vacuum packaging than MAP (Subbaraj et al., 2016), which indicates packaging effects on metabolite levels.
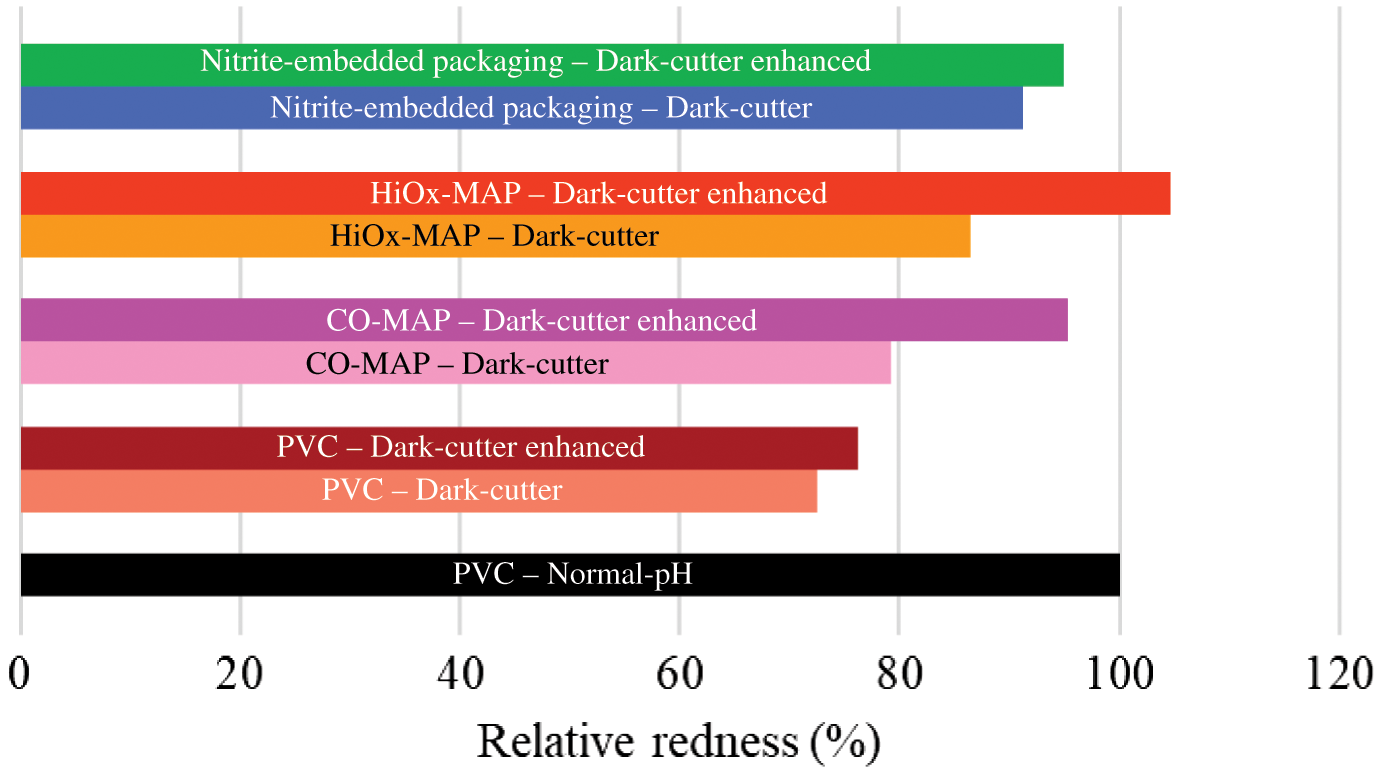
Altering gas compositions within the package improved the appearance of high-pH meat (Mitacek et al., 2018; Zhang et al., 2018). A greater pH promotes muscle swelling and oxygen-consuming enzyme activity, which can decrease light reflectance and oxygenation, respectively. Hence, acidification (to counteract the effect of high pH) and modifying gas compositions (to meet oxygen demand or form carboxymyoglobin) within a package are the 2 major approaches that have been used to improve the appearance of high-pH beef. Acidification alone has limited success to enhance the color of dark-cutting beef in PVC packaging (improved redness by 8% compared to control dark-cutting steaks; Stackhouse et al., 2016). However, modifying gas composition using HiOx-MAP or CO-MAP had a significant impact on dark-cutting beef color. Previous research demonstrated that dark-cutting beef had greater deoxymyoglobin content in bloomed steaks than control (English et al., 2016a). This suggests that oxygen utilization is greater in dark-cutting steaks than normal-pH steaks. Packaging dark-cutting steaks in HiOx-MAP and CO-MAP improved redness compared with dark-cutting steaks in PVC (Figure 5). However, a combined effect of rosemary enhancement with high-oxygen packaging resulted in greater redness than dark-cutting steaks packaged in HiOx-MAP (without enhancement; Wills et al., 2017). Improved color by rosemary enhancement can be attributed to water present in enhancement solution and deeper penetration of oxygen into the meat. More specifically, water can improve reflectance and also serves as a medium to provide dissolved oxygen to myoglobin. Redness of dark-cutting steaks in CO-MAP was lower than normal-pH steaks in CO-MAP. This suggests that greater muscle swelling may have limited CO penetration inside dark-cutting steak compared with normal-pH steak. As indicated in Figure 5, HiOx-MAP was more effective in improving color than CO-MAP. For each molecule of myoglobin on the surface of the steak, 1.87 moles of CO are present within CO-MAP, and 370 moles of oxygen are present within a package (calculation included in the supporting information). Hence, fewer CO molecules are available to bind with dark-cutting steaks.
Effects of packaging on redness of dark-cutting beef. Relative redness (a* values) compared with normal-pH steak packaged in PVC is presented. The results based on Wills et al. 2017 and Ramanathan et al., 2018. CO-MAP = carbon monoxide modified atmospheric packaging; HiOx-MAP = high-oxygen modified atmospheric packaging; PVC = polyvinyl chloride.
Nitric oxide myoglobin also can impart bright-red color. The use of patented nitrite-embedded packaging improved the redness of dark-cutting steak (Ramanathan et al., 2018). The formation of nitric oxide myoglobin involves the conversion of oxymyoglobin (form present on the surface of steak before packaging) to deoxymyoglobin. However, during this conversion, metmyoglobin is formed, and the length of metmyoglobin within the package depends on the reducing activity of meat. High-pH steaks have greater metmyoglobin reducing activity than normal-pH steaks. Hence, bright-red nitric oxide formation was rapid in dark-cutting beef compared with normal-pH beef.
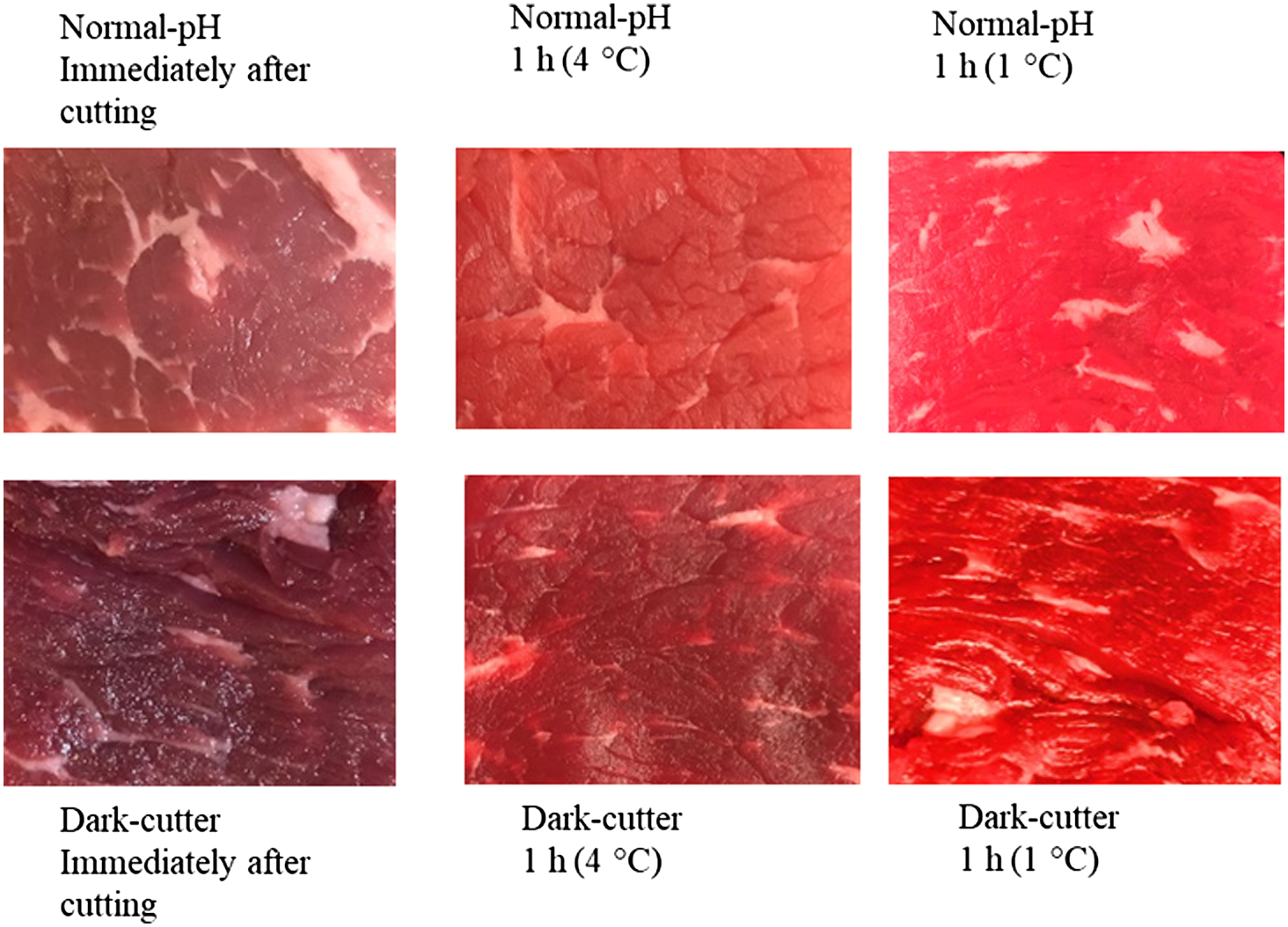
The temperature has a critical influence on the activity of mitochondrial and oxygen-consuming enzymes (Tang et al., 2005). Figure 6 shows that the blooming of both normal-pH meat and dark-cutting beef increased at lower temperatures. More specifically, a lower temperature limits the activity of mitochondria and can saturate myoglobin with available oxygen. Previous research noted that redness of prerigor muscle was improved by the addition of rotenone, a mitochondrial inhibitor (Cornforth and Egbert, 1985). Therefore, actively respiring or inactive mitochondria may not contribute to a desirable bright-red color, but moderately active mitochondria can help provide oxymyoglobin. In summary, various postharvest processes such as enhancement and MAP have the potential to improve the color of dark-cutting beef.
Effect of pH and temperature on blooming properties of beef longissimus lumborum steaks. (a) Initial/no bloom of normal-pH beef (pH = 5.6) to partial bloom in 1 h at 4°C where oxygen consumption is competing with myoglobin, and best bloom for 1 h at 1°C, where oxygen consumption enzymes are less active, making more oxygen available to the myoglobin. Loins were vacuum packaged and stored for 14 d at 2°C. (b) Initial/no bloom of dark-cutting beef (pH = 6.4) to slight bloom at 4°C 1 h. Dark-cutting beef bloomed 1 h at 1°C had more bloom due to less oxygen consumption from enzyme activity. Loins were vacuum packaged and stored for 14 d at 2°C (Ramanathan, Denzer, and Mafi, unpublished data, 2020).
Muscle-Specific Differences in Color Stability
The skeletal muscular system in animals is primarily responsible for locomotion via the contraction and relaxation of muscle. Within a species and anatomical location, the skeletal muscle cells vary in their physical structure and their biochemical, physiological, and histochemical properties. For example, Type I fibers (also known as red, more aerobic fibers) are usually smaller in diameter; are slower to fatigue; contract slower; and have greater capillary density, mitochondria, and myoglobin content than Type II fibers. Type II fibers (also known as white fibers) have a larger diameter and are often divided into 2 types. Type IIb are larger in diameter than Type I fibers, have more glycolytic metabolism, have fewer mitochondria, have less myoglobin, and contract and fatigue faster than Type I fibers. Type IIa fibers are often more intermediate fibers both physiologically and biochemically. Depending on the physiological function of the muscle, the percentages of each fiber type are specific to each muscle. In addition, anatomical location can also influence fiber type. More specifically, the population density of fiber type varies from the outer, more superficial part of the muscle, which is more “white,” to the deep, more inner location, which is more “red.”
The locational patterns of fiber types can affect muscle prerigor chemistry including the rate of pH decline and ultimate pH that affect the dynamics of meat color and the stability of the color during meat processing. McKenna et al. (2005) characterized 19 bovine muscles and classified muscles as high, moderate, intermediate, low, and very low color stability based on discoloration during retail display. Biochemical factors such as metmyoglobin reducing activity, oxygen consumption, lipid oxidation, and oxygen penetration can explain some of the differences in color stability among muscles and/or animals. Muscles with contrasting color stability characteristics such as color-stable longissimus and color-labile psoas muscles are often used to elucidate muscle-specific differences in meat color chemistry. Psoas muscle discolors within 3 d of retail display, while longissimus retains color during display. Psoas muscle has predominant red fibers compared with longissimus muscles. Accordingly, psoas muscles have greater oxygen consumption than the longissimus. This suggests that oxidative changes are greater in psoas muscles than longissimus. Because mitochondrial function is closely related to meat color, research determining muscle-specific differences in mitochondrial properties indicated that psoas has greater mitochondrial content than longissimus muscles (Mohan et al., 2010c; Ke et al., 2017). Isolated psoas mitochondria have a greater oxygen consumption rate than longissimus mitochondria. However, mitochondrial degeneration was greater in psoas muscle than longissimus muscles. Cytochrome c is an electron carrier within mitochondria, and its presence in sarcoplasm is used as an indicator of mitochondria damage. By the end of display time, more cytochrome c was in the sarcoplasm of psoas muscles than longissimus.
Many studies report that color stability is muscle specific for differences in oxygen consumption and metmyoglobin reducing activity. However, King et al. (2011) reported it is not always possible to determine whether superior muscle color stability is due strictly to muscle differences between these 2 key factors of color stability. They posed a critical question—are the muscle-specific differences valid when comparing animal-to-animal variation of color stability for a given muscle? Their data indicated that the timing of assays for these 2 traits were very important. They compared 257 longissimus steaks (one of the best color stability muscles) during 6 d of display. The initial capacity for oxygen consumption when combined with both the initial metmyoglobin reducing activity and the metmyoglobin reducing activity at the termination of display were best indicators of animal variation in color stability.
In recent years, a systems biology approach utilizing omics technology such as proteomics, metabolomics, and genomics has been incorporated into basic meat science research to characterize the relative abundance of proteins or metabolites. Several studies have successfully incorporated omics techniques in meat color research to understand the mechanistic basis for discoloration (Table 4). Metabolomics research indicated that glycolytic compounds (fructose, glucose 6-phosphate, and pyruvic acid) were more abundant in longissimus lumborum than psoas major muscles (Abraham et al., 2017). Further, citrate levels were greater in longissimus than psoas muscles by the end of the 6 d retail display. In support, proteomics studies have indicated the overabundance of pyruvate dehydrogenase in color-stable muscles (Joseph et al., 2012). This suggests that, in longissimus, pyruvate may be entering the Krebs cycle and thereby increasing citrate levels. Furthermore, color-labile psoas had a higher mitochondrial aconitase level compared with color-stable longissimus, which may be another reason for the lower level of citrate in psoas major muscles. Metabolomics research is supported by the proteomics study on longissimus and psoas muscles. For example, Joseph et al. (2012) reported heat shock proteins, antioxidant proteins β-enolase, and triose phosphate isomerase (glycolytic enzymes) were greater in color-stable longissimus than in color-labile psoas muscles. In another study, color-stable ovine muscles had greater NADH, malic acid, and guanosine levels than color-labile muscles (Subbaraj et al., 2016). All this research indicated that the inherent alterations in muscle biochemistry could lead to a difference in the color stability of muscles during retail display.
Summary of major proteomics, genomics, and metabolomics research related to meat color in the last 15 years
| Title | Technique | Key findings | Reference |
|---|---|---|---|
| Proteomics | |||
| Proteome analysis of the sarcoplasmic fraction of pig semimembranosus muscle: Implications on meat color development | 2-dimensional gel electrophoresis + MS | Enzymes related to glycolysis were over-expressed in pigs with lighter color | Sayd et al., 2006 |
| Proteomics of muscle-specific beef color stability | 2-dimensional gel electrophoresis + MS | Color-stable longissimus demonstrated greater abundance of metabolic enzymes, antioxidant proteins, and chaperones compared with color-labile psoas | Joseph et al., 2012 |
| Changes in meat quality traits and sarcoplasmic proteins during aging in three different cattle breeds | 2-dimensional gel electrophoresis + MS | The proteome changes were associated with variation in beef quality between breeds during aging | Marino et al., 2014 |
| Understanding early post-mortem biochemical processes underlying meat color and pH decline in the longissimus thoracis muscle of young blond d’aquitaine bulls using protein biomarkers | Western blot and Dot-blot | Determined relationship between protein | Gagaoua et al., 2015 |
| Towards muscle-specific meat color stability of Chinese Luxi yellow cattle: A proteomic insight into post-mortem storage | 2-dimensional gel electrophoresis + MS | Glycolytic and antioxidant proteins were expressed differentially between longissimus and psoas | Wu et al., 2016 |
| Post-mortem changes in sarcoplasmic proteome and its relationship to meat color traits in M. semitendinosus of Chinese Luxi yellow cattle | 2-dimensional gel electrophoresis + MS | Triosephosphate isomerase, L-lactate dehydrogenase A chain isoform, fructose-bisphosphate aldolase A isoform, peroxiredoxin-6, and pyruvate kinase isozymes M1/M2 isoform were highly related to meat color parameters | Wu et al., 2015 |
| Differential abundance of sarcoplasmic proteome explains animal effect on beef Longissimus lumborum color stability | 2-dimensional gel electrophoresis + MS | Metabolic enzymes differentially abundant in color-stable longissimus steaks exhibited a positive correlation with color stability | Canto et al., 2015 |
| Proteome basis for intramuscular variation in color stability of beef semimembranosus | 2-dimensional gel electrophoresis + MS | Inside semimembranosus steaks had greater abundance of glycolytic enzymes than their outside semimembranosus counterparts | Nair et al., 2016 |
| Proteomic and peptidomic differences and similarities between four muscle types from New Zealand raised Angus steers | 2-dimensional difference gel electrophoresis + MS | The predominant oxidative metabolism in dark muscles delays postmortem pH fall and offers protection against protein denaturation | Clerens et al., 2016 |
| Proteome basis of pale, soft, and exudative-like (PSE-like) broiler breast (Pectoralis major) meat | 2-dimensional gel electrophoresis + MS | Overabundance of proteins involved in glycolytic pathways, muscle contraction, proteolysis, ATP regeneration, and energy metabolism in PSE breast could be related to the quality differences between normal and PSE meat | Desai et al., 2016 |
| Comparative proteomics to reveal muscle-specific beef color stability of Holstein cattle during post-mortem storage | Label-free MS | Proteins related to oxidation-reduction processes, TCA cycle, and electron transport chain processes could contribute to muscle-specific beef color stability | Yu et al., 2017b |
| Unraveling proteome changes of Holstein beef M. semitendinosus and its relationship to meat discoloration during post-mortem storage analyzed by label-free mass spectrometry | Label-free MS | Eighteen differentially abundant proteins were correlated closely with the a* value | Yu et al., 2017a |
| The study of protein biomarkers to understand the biochemical processes underlying beef color development in young bulls | Dot-blot | Determined the relationship between biomarkers, meat color, and metabolic processes | Gagaoua et al., 2017 |
| Changes in the sarcoplasmic proteome of beef muscles with differential color stability during postmortem aging | 2-dimensional gel electrophoresis + MS | Glycolytic enzymes were more abundant in color-stable muscles and at aging times with greater color stability | Nair et al., 2018 |
| Intramuscular variations in color and sarcoplasmic proteome of beef semimembranosus during postmortem aging | 2-dimensional gel electrophoresis + MS | Effect of aging on sarcoplasmic proteome of beef semimembranosus was influenced by the location within the muscle | Nair et al., 2018 |
| Quantitative phosphoproteomic analysis among muscles of different color stability using tandem mass tag labeling | Tandem mass tag labeling with TiO2 phospho-peptide enrichment | Among the differentially phosphorylated proteins, 27 phosphoproteins were key color-related proteins, including glycolytic enzymes and myoglobin | Li et al., 2018 |
| Proteomic analysis to investigate color changes of chilled beef longissimus steaks held under carbon monoxide and high oxygen packaging | 2-dimensional gel electrophoresis | Glycolytic and energy metabolic enzymes important in NADH regeneration and antioxidant processes were more abundant in carbon monoxide–modified atmospheric packaged steaks (0.4% CO) | Yang et al., 2018 |
| Metabolomics | |||
| CE-TOF MS-based metabolomic profiling revealed characteristic metabolic pathways in postmortem porcine fast and slow type muscles | Capillary electrophoresis-time of flight MS | AMP, IMP, and inosine changed differently between muscles during postmortem aging | Muroya et al., 2014 |
| A hydrophilic interaction liquid chromatography–mass spectrometry (HILIC–MS) based metabolomics study on color stability of ovine meat | Hydrophilic interaction liquid chromatography–MS | Compounds with antioxidant properties were found in higher levels in color-stable samples | Subbaraj et al., 2016 |
| Metabolite profile differences between beef longissimus and psoas muscles during display | Gas chromatography–MS | Inherent metabolite differences contribute to the muscle-specific beef color stability | Abraham et al., 2017 |
| Metabolomics profiling to determine the effect of postmortem aging on color and lipid oxidative stabilities of different bovine muscles | High-performance liquid chromatography–MS | Color and oxidative stability could be associated with aging, but are also muscle-specific | Ma et al., 2017 |
| Genomics | |||
| Investigation of quantitative trait locus (QTL) regions on chromosome 17 for genes associated with meat color in the pig | Linkage mapping, QTL mapping, and association | EDN3 and PHACTR3 genes could potentially affect meat color in pigs | Fan et al., 2008 |
| Detection of a Cis eQTL controlling BMCO1 gene expression leads to the identification of a QTG for chicken breast meat color | QTL analysis and gene eQTL | Two fully-linked SNPs located within the proximal promoter of the BCMO1 gene were identified | Le Bihan-Duval et al., 2011 |
| Association of polymorphisms at DGAT1, leptin, SCD1, CAPN1 and CAST genes with color, marbling and water holding capacity in meat from beef cattle populations in Sweden | Real-time PCR system with TaqMan chemistry and probes | SNPs at the leptin, SCD1, and CAPN1 genes were associated with meat color | Li et al., 2013 |
| Genome-wide association of meat quality traits and tenderness in swine | Illumina PorcineSNP60 BeadChip | Three associations were found for L* value and one association for b* value, whereas no associations were detected for a* value | Nonneman et al., 2013 |
| A genome-wide association study of production traits in a commercial population of Large White pigs: Evidence of haplotypes affecting meat quality | PorcineSNP60 BeadChip | SSC1a region and SSC9a region had moderate effects on meat color, whereas SSC13 QTL had an effect of 0.75 STD on meat lightness | Sanchez et al., 2014 |
| Genome-wide association of myoglobin concentrations in pork loins | Bayes-Cpi using GenSel and Bayes-C variable selection method | Potential biomarkers that could be used to select for improved lean meat color in pork were identified | Cross et al., 2018 |
| Genomic selection for meat quality traits in Nelore cattle | Methods of gBLUP, improved Bayesian Lasso and Bayes Cπ | The estimates of heritability for meat quality traits were low to moderate | Magalhães et al., 2019 |
| Meat quality of the longissimus lumborum muscle of Casertana and Large White pigs: Metabolomics and proteomics intertwined | Rapid resolution reversed-phase HPLC and ESI MS | Breed-specific differences at the protein level were related to growth performances and fat accumulation tendency, and postmortem performances | D’Alessandro et al., 2011 |
AMP = adenosine monophosphate; ATP = adenosine triphosphate; eQTL = expression QTL; ESI = electron spray ionization; gBLUP = genomic best linear unbiased prediction; HPLC = high performance liquid chromatography; IMP = inosine monophosphate; MS = mass spectrometry; NADH = nicotinamide adenine dinucleotide reduced form; PSE = pale, soft, and exudative; QTL = quantitative trait locus; SNP = single-nucleotide polymorphism; STD =; TCA = tricarboxylic acid.
The beef semimembranosus is a larger muscle that shows significant differences in color stability between outside and inside portions (Sammel et al., 2002). Hence, semimembranosus can be used as a model to study intramuscular variation in color. For example, the outside portion of the semimembranosus is more color stable compared with the inside semimembranosus. Even though the inner portion often has greater initial redness than the outer portion, the inner portion discolors more rapidly during display. The temperature difference between outside (faster chilling) and inside (slower chilling) may partially explain variations in intramuscular metabolism resulting in a more rapid decline at greater temperature resulting in greater protein denaturation and lower water-holding capacity typical of pale, soft, and exudative beef. Proteomics research demonstrated that the inner portion of the semimembranosus steaks had a greater abundance of glycolytic enzymes than their outside semimembranosus counterparts (Nair et al., 2016). Furthermore, outside semimembranosus demonstrated greater mitochondrial activity than inside semimembranosus by the end of the 4-d display (Nair et al., 2017b). Muscles with more glycolytic fibers are more color stable than muscles with oxidative fibers, which may explain animal-to-animal variation within longissimus muscles. For example, color-stable longissimus had a greater abundance of glycolytic enzymes than less color-stable counterparts. Raines et al. (2010) utilized various cow muscles categorized as having high, intermediate, or low color stability to study the color stability of ground beef. Various blends of trim could be used without adversely affecting display color stability, provided that at least 50% of the trim was from high–color-stability muscle and that the low–color-stability trim did not exceed 25%. In summary, muscle fiber type, to a certain extent, can explain muscle-specific differences in color stability.
Future Directions in Color Research
Color measurement methodology
Most published methods use surface reflectance/absorbance spectrophotometers or colorimeters to determine meat’s surface color and relative amounts of myoglobin forms (Table 5). Deviation in ultimate muscle pH can affect reflectance and absorbance properties. A comparison of 2 commonly used methodologies, ratio between absorption coefficient and scattering coefficient (K/S) (AMSA, 2012) versus reflex attenuance (Krzywicki, 1979), revealed that the two varied considerably in absolute amounts of redox forms. The K/S method had more “realistic” amounts of the redox forms, but both methods were highly correlated (R2 = 0.87 for deoxymyoglobin, R2 = 0.97 for oxymyoglobin, and R2 = 0.94 for metmyoglobin). Thus, the authors concluded that both were equivalent for statistical decision making (Hernández et al., 2015). Another color measurement technique (Khatri et al., 2012) reported improved precision and accuracy for deoxymyoglobin, oxymyoglobin, and metmyoglobin using a spectrum from 400 to 1,100 nm combined with complex data handling and statistical analyses. Recently, a computer vision system has been utilized in color measurements (Tomasevic et al., 2019a). A summary of various approaches, advantages, and limitations is given in Table 5. Previous studies reported myoglobin forms, oxygen consumption, and metmyoglobin reducing activity as a ratio or percentage. There are several advanced techniques such as an oxygen probe, near-infrared (NIR)-based techniques, or sensors, which can be utilized in meat science research to create a benchmark for various biochemical values. Use of a unified approach will help researchers around the globe to relatively compare results or determine the effects of various factors on meat color. There is a need for improved meat color measurement methodology.
Different approaches, advantages, and limitations of meat color measurements
| Method | Advantage | Limitation | Reference |
|---|---|---|---|
| Reflectance and absorbance methods using colorimeter such as HunterLab (HunterLab, Reston, VA) or Minolta or integrated sphere spectrophotometer | Easy and multiple readings can be recorded | Measure only surface color and will not record color changes beneath the surface | AMSA, 2012 |
| L*, a*, and b* values | Easy to record and interpret | Will not provide information about myoglobin redox forms | |
| K/S ratio | Takes into account absorbance and scattering from the surface | Need to make standards in order to quantify myoglobin forms on the surface | |
| Absorbance | Need to extract myoglobin forms by homogenization and centrifugation | A larger sample number makes extraction challenging and deoxymyoglobin cannot be quantified | Krzywicki, 1979 |
| Nix colorimeter | Smaller, hand-held, uses smartphone application to measure color, more portable and tolerant of cooler conditions | Will not provide information about redox forms and less accurate | Holman et al., 2018a |
| Partial least-square regression | Less error than K/S method using standards to quantify myoglobin forms on steak surface | Requires knowledge in modeling | Khatri et al., 2012 |
| NIR | Can determine subsurface color chemistry | Required standards for OMb and DMb. The concentration of MMb was estimated as [(100-(OMb + DMb)] | Mohan et al., 2010a |
| Computer vision system | More sensitive than colorimeter such as HunterLab or Minolta and can cover larger area | Comparison between laboratory can be challenging as there are no standardized conditions for camera and data analysis | Tomasevic et al., 2019b |
| Diffuse reflectance spectroscopy | More accurate than reflectance approach | Need data processing | Nguyen et al., 2019 |
K/S = absorption and scattering coefficient; NIR = near-infrared.
Mechanism of discoloration
The classical meat discoloration progressing from a bright-red color to tannish-brown metmyoglobin results from a subsurface interface where the upper bound is at an oxygen partial pressure that causes the auto-oxidation to metmyoglobin and the lower bound is where the partial pressure is approaching zero, resulting in deoxymyoglobin. As time passes, the brown layer works its way to the surface. To understand the dynamics of the migration, refinement of new methodology is needed. Hence, the development of noninvasive techniques to quantify myoglobin forms is important to understand meat color chemistry. NIR spectroscopy has the potential to determine color changes beneath the surface. The NIR-based approach has been utilized to study myoglobin redox forms and to determine hemoglobin oxygen saturation in athletes. Both myoglobin and hemoglobin have similar spectral characteristics, and meat science research has utilized the NIR-based approach to determine myoglobin oxygenation properties. NIR-based approaches are expensive and require standardization. Furthermore, most researchers utilize changes in myoglobin redox forms to determine metmyoglobin reducing activity and oxygen consumption. Adopting techniques from biomedical research to determine the absolute amount of oxygen consumption and reducing activity in tissue can provide additional insights into color chemistry.
A systematic analysis of metabolites that can influence metmyoglobin reducing activity and oxygen consumption will provide more insights into meat color. For example, enhancing meat with ingredients such as lactate, malate, or pyruvate has shown substrate-specific influence on meat color. Pyruvate-enhanced beef longissimus steaks in vacuum package discolored, while pyruvate-enhanced steaks packaged in HiOx had improved color compared with nonenhanced control steaks. These studies reconfirm the role of metabolites in meat color. Although injection enhancement of ingredients in fresh beef is not as common as in the past, these studies clearly show that postmortem muscle is biochemically active and offers the possibility of functional ingredients being a part of feeding regimens, packaging materials, or nanotechnology as they emerge into new eras of fresh meat marketing. Certainly, more attention should be paid to the timing of assays, especially for when and how oxygen consumption and metmyoglobin reducing activity and reducing capacity relate to variation of animal-to-animal color stability.
There are numerous qualitative and quantitative methods for characterizing meat at a given point in time. But few, if any, of these traits are predictive of meat’s future performance for color, color stability, or any other value-based trait related to meat color. Surely some live animal selection pressure can be applied to animal production as some meat color traits are reasonably heritable (King et al., 2010).
Conclusions
With a major thrust on the environment and limiting food waste, the meat industry has to be creative in improving the colorlife and shelflife of meat products to address the issues associated with discoloration and discarded products in the value chain. New or improved ways to create the desired bright-red color of meat are needed. Furthermore, novel ways to help control all factors affecting the desired color and colorlife are needed. This also includes the potential use of biodegradable packaging materials and the application of active packaging to improve meat quality. Meat science researchers and industry may consider adopting some of the approaches such as NIR or sensor-based techniques utilized in biomedical or exercise physiology to determine myoglobin redox forms, oxygen partial pressure, and reduction potential. Understanding fundamental biochemical characteristics such as metmyoglobin reducing activity, oxygen diffusion into the meat, and oxygen consumption is important to elucidate the meat discoloration process. The knowledge gained from fundamental research may be combined with artificial intelligence and machine learning to predict color-stable muscles and/or can be used in the meat processing production line to maximize the value and to limit the waste of nutritionally dense meat.
Literature Cited
Abraham, A., J. W. Dillwith, G. G. Mafi, D. L. VanOverbeke, and R. Ramanathan. 2017. Metabolite profile differences between beef longissimus and psoas muscles during display. Meat Muscle Biology. 1:18–27. https://doi.org/10.22175/mmb2016.12.0007.
Adzitey, F., and H. Nurul. 2011. Pale soft exudative (PSE) and dark firm dry (DFD) meats: Causes and measures to reduce these incidences—A mini review. Int. Food Res. J. 18:11–20.
Alderton, A. L., C. Faustman, D. C. Liebler, and D. W. Hill. 2003. Induction of redox instability of bovine myoglobin by adduction with 4-hydroxy-2-nonenal. Biochemistry. 42:4398–4405. https://doi.org/10.1021/bi0271695.
Allen, K., and D. Cornforth. 2010. Comparison of spice-derived antioxidants and metal chelators on fresh beef color stability. Meat Sci. 85:613–619. https://doi.org/10.1016/j.meatsci.2010.03.012.
AMSA. 2012. AMSA meat color measurement guidelines. Am. Meat Sci. Assoc, Champaign, Illinois. 2nd edition. p. 1–135.
Apaoblaza, A., S. D. Gerrard, S. K. Matarneh, J. C. Wicks, L. Kirkpatrick, E. M. England, T. L. Scheffler, S. K. Duckett, H. Shi, S. L. Silva, A. L. Grant, and D. E. Gerrard. 2020. Muscle from grass- and grain-fed cattle differs energetically. Meat Sci. 161:107996. https://doi.org/10.1016/j.meatsci.2019.107996.
Bak, K. H., T. Bolumar, A. H. Karlsson, G. Lindahl, and V. Orlien. 2019. Effect of high pressure treatment on the color of fresh and processed meats: A review. Crit. Rev. Food Sci. Nutr. 59:228–252. https://doi.org/10.1080/10408398.2017.1363712.
Balentine, C. W., P. G. Crandall, C. A. O’Bryan, D. Q. Duong, and F. W. Pohlman. 2006. The pre- and post-grinding application of rosemary and its effects on lipid oxidation and color during storage of ground beef. Meat Sci. 73:413–421. https://doi.org/10.1016/j.meatsci.2005.12.003.
Bao, H. N. D., H. Ushio, and T. Ohshima. 2008. Antioxidative activity and antidiscoloration efficacy of ergothioneine in mushroom (Flammulina velutipes) extract added to beef and fish meats. J. Agr. Food Chem. 56:10032. https://doi.org/10.1021/jf8017063.
Battisti, R., N. Fronza, Á. Vargas Júnior, S. M. D. Silveira, M. S. P. Damas, and M. G. N. Quadri. 2017. Gelatin-coated paper with antimicrobial and antioxidant effect for beef packaging. Food Packaging and Shelf Life. 11:115–124. https://doi.org/10.1016/j.fpsl.2017.01.009.
Bendall, J. R., and A. A. Taylor. 1972. Consumption of oxygen by the muscles of beef animals and related species. II. Consumption of oxygen by post-rigor muscle. J. Sci. Food Agr. 23:707–719. https://doi.org/10.1002/jsfa.2740230606.
Bouarab-Chibane, L., B. Ouled-Bouhedda, L. Leonard, L. Gemelas, J. Bouajila, H. Ferhout, A. Cottaz, C. Joly, P. Degraeve, and N. Oulahal. 2017. Preservation of fresh ground beef patties using plant extracts combined with a modified atmosphere packaging. Eur. Food Res. Technol. 243:1997–2009. https://doi.org/10.1007/s00217-017-2905-3.
Brennecke, K. R, R. M. Mitacek, R. A. Mancini, K. Bailey, G. G. Mafi, D. L. VanOverbeke, and R. Ramanathan. 2017. Effects of lactate-enhancement on beef muscle structure. Presented at the Institute of Food Technologists Annual Meeting, Las Vegas, Nevada, June 25–28.
Brewer, S. 2004. Irradiation effects on meat color - A review. Meat Sci. 68:1–17. https://doi.org/10.1016/j.meatsci.2004.02.007.
Brooks, J. C., M. Alvarado, T. P. Stephens, J. D. Kellermeier, A. W. Tittor, M. F. Miller, and M. M. Brashears. 2008. Spoilage and safety characteristics of ground beef packaged in traditional and modified atmosphere packages. J. Food Protect. 71:293–301. https://doi.org/10.4315/0362-028X-71.2.293.
Camo, J., J. A. Beltrán, and P. Roncalés. 2008. Extension of the display life of lamb with an antioxidant active packaging. Meat Sci. 80:1086–1091. https://doi.org/10.1016/j.meatsci.2008.04.031.
Canto, A. C. V. C. S., S. P. Suman, M. N. Nair, S. Li, G. Rentfrow, C. M. Beach, T. J. P. Silva, T. L. Wheeler, S. D. Shackelford, A. Grayson, R. O. McKeith, and D. A. King. 2015. Differential abundance of sarcoplasmic proteome explains animal effect on beef Longissimus lumborum color stability. Meat Sci. 102:90–98. https://doi.org/10.1016/j.meatsci.2014.11.011.
Carpenter, C. E., D. P. Cornforth, and D. Whittier. 2001. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 57:359–363. https://doi.org/10.1016/S0309-1740(00)00111-X.
Carpenter, R., M. N. O’Grady, Y. C. O’Callaghan, N. M. O’Brien, and J. P. Kerry. 2007. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 76:604–610. https://doi.org/10.1016/j.meatsci.2007.01.021.
Cheung, R., P. McMahon, E. Norell, R. Kissel, and D. B. 2017. Back to grass: The market potential for grassfed beef. https://www.stonebarnscenter.org/wp-content/uploads/2017/10/Grassfed_Full_v2.pdf. (Accessed 24 March 2020).
Claus, J. R., and C. Du. 2013. Nitrite-embedded packaging film effects on fresh and frozen beef color development and stability as influenced by meat age and muscle type. Meat Sci. 95:526–535. https://doi.org/10.1016/j.meatsci.2013.05.029.
Clerens, S., A. Thomas, J. Gathercole, J. E. Plowman, T. Y. Yu, A. J. Grosvenor, S. R. Haines, P. Dobbie, K. Taukiri, K. Rosenvold, J. M. Dyer, and S. Deb-Choudhury. 2016. Proteomic and peptidomic differences and similarities between four muscle types from New Zealand raised Angus steers. Meat Sci. 121:53–63. https://doi.org/10.1016/j.meatsci.2016.05.014.
Cornforth, D. P., and W. R. Egbert. 1985. Effect of rotenone and pH on the color of pre-rigor muscle. J. Food Sci. 50:34–35. https://doi.org/10.1111/j.1365-2621.1985.tb13271.x.
Cross, A. J., D. A. King, S. D. Shackelford, T. L. Wheeler, D. J. Nonneman, B. N. Keel, and G. A. Rohrer. 2018. Genome-wide association of myoglobin concentrations in pork loins. Meat Muscle Biology. 2:189. https://doi.org/10.22175/mmb2017.08.0042.
Djenane, D., J. Beltrán, J. Camo, and P. Roncalés. 2016. Influence of vacuum-ageing duration of whole beef on retail shelf life of steaks packaged with oregano (Origanum vulgare L.) active film under high O2. J. Food Sci. Technol. 53:4244–4257. https://doi.org/10.1007/s13197-016-2419-1.
Djenane, D., L. Martínez, A. Sánchez-Escalante, J. A. Beltrán, and P. Roncalés. 2004. Antioxidant effect of carnosine and carnitine in fresh beef steaks stored under modified atmosphere. Food Chem. 85:453–459. https://doi.org/10.1016/j.foodchem.2003.08.007.
Djenane, D., A. Sánchez-Escalante, J. A. Beltrán, and P. Roncalés. 2002. Ability of α-tocopherol, taurine and rosemary, in combination with vitamin C, to increase the oxidative stability of beef steaks packaged in modified atmosphere. Food Chem. 76:407–415. https://doi.org/https://doi.org/10.1016/S0308-8146(01)00286-2.
D’Alessandro, A., C. Marrocco, V. Zolla, M. D’Andrea, and L. Zolla. 2011. Meat quality of the longissimus lumborum muscle of Casertana and Large White pigs: Metabolomics and proteomics intertwined. J. Proteomics. 75:610–627. https://doi.org/10.1016/j.jprot.2011.08.024.
Desai, M. A., V. Jackson, W. Zhai, S. P. Suman, M. N. Nair, C. M. Beach, and M. W. Schilling. 2016. Proteome basis of pale, soft, and exudative-like (PSE-like) broiler breast (Pectoralis major) meat. Poultry Sci. 95:2696–2706. https://doi.org/10.3382/ps/pew213.
Donaldson, A. E., and I. L. Lamont. 2013. Biochemistry changes that occur after death: Potential markers for determining post-mortem interval. PLoS One. 8:e82011. https://doi.org/10.1371/journal.pone.0082011.
English, A. R., G. G. Mafi, D. L. Vanoverbeke, and R. Ramanathan. 2016a. Effects of extended aging and modified atmospheric packaging on beef top loin steak color. J. Anim. Sci. 94:1727–1737. https://doi.org/10.2527/jas.2015-0149.
English, A. R., K. M. Wills, B. N. Harsh, G. G. Mafi, D. L. VanOverbeke, and R. Ramanathan. 2016b. Effects of aging on the fundamental color chemistry of dark-cutting beef. J. Anim. Sci. 94:4040–4048. https://doi.org/10.2527/jas2016-0561.
Esmer, O. K., R. Irkin, N. Degirmencioglu, and A. Degirmencioglu. 2011. The effects of modified atmosphere gas composition on microbiological criteria, color and oxidation values of minced beef meat. Meat Sci. 88:221–226. https://doi.org/10.1016/j.meatsci.2010.12.021.
Fan, B., K. L. Glenn, B. Geiger, A. Mileham, and M. F. Rothschild. 2008. Investigation of QTL regions on Chromosome 17 for genes associated with meat color in the pig. J. Anim. Breed. Genet. 125:240–247. https://doi.org/10.1111/j.1439-0388.2008.00749.x.
Faustman, C., Q. Sun, R. Mancini, and S. P. Suman. 2010. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 86:86–94. https://doi.org/10.1016/j.meatsci.2010.04.025.
Gagaoua, M., E. M. C. Terlouw, D. Micol, A. Boudjellal, J. F. Hocquette, and B. Picard. 2015. Understanding early post-mortem biochemical processes underlying meat color and pH decline in the longissimus thoracis muscle of young blond dÁquitaine bulls using protein biomarkers. J. Agr. Food Chem. 63:6799–6809. https://doi.org/10.1021/acs.jafc.5b02615.
Gagaoua, M., E. M. C. Terlouw, and B. Picard. 2017. The study of protein biomarkers to understand the biochemical processes underlying beef color development in young bulls. Meat Sci. 134:18–27. https://doi.org/10.1016/j.meatsci.2017.07.014.
George, P., and C. J. Stratmann. 1952. The oxidation of myoglobin to metmyoglobin by oxygen. I. Biochem. J. 51:103–108.
Guelker, M. R., A. N. Haneklaus, J. C. Brooks, C. C. Carr, R. J. Delmore, D. B. Griffin, D. S. Hale, K. B. Harris, G. G. Mafi, D. D. Johnson, C. L. Lorenzen, R. J. Maddock, J. N. Martin, R. K. Miller, C. R. Raines, D. L. Vanoverbeke, L. L. Vedral, B. E. Wasser, and J. W. Savell. 2013. National beef tenderness survey-2010: Warner-Bratzler shear force values and sensory panel ratings for beef steaks from United States retail and food service establishments. J. Anim. Sci. 91:1005–1014. https://doi.org/10.2527/jas.2012-5785.
Garrido, M. D., M. Auqui, N. Martí, and M. B. Linares. 2011. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT-Food Sci. Technol. 44:2238–2243. https://doi.org/10.1016/j.lwt.2011.07.003.
Georgantelis, D., G. Blekas, P. Katikou, I. Ambrosiadis, and D. J. Fletouris. 2007. Effect of rosemary extract, chitosan and α-tocopherol on lipid oxidation and colour stability during frozen storage of beef burgers. Meat Sci. 75:256–264. https://doi.org/10.1016/j.meatsci.2006.07.018.
Gill, C. O., and J. C. McGinnis. 1995. The use of oxygen scavengers to prevent the transient discolouration of ground beef packaged under controlled, oxygen-depleted atmospheres. Meat Sci. 41:19–27. https://doi.org/10.1016/0309-1740(94)00064-E.
Grobbel, J., M. Dikeman, M. Hunt, and G. Milliken. 2008a. Effects of different packaging atmospheres and injection-enhancement on beef tenderness, sensory attributes, desmin degradation, and display color. J. Anim. Sci. 86:2697–2710. https://doi.org/10.2527/jas.2007-0824.
Grobbel, J. P., M. E. Dikeman, M. C. Hunt, and G. A. Milliken. 2008b. Effects of packaging atmospheres on beef instrumental tenderness, fresh color stability, and internal cooked color. J. Anim. Sci. 86:1191. https://doi.org/10.2527/jas.2007-0479.
Hayes, J. E., V. Stepanyan, P. Allen, M. N. O’Grady, and J. P. Kerry. 2010. Effect of lutein, sesamol, ellagic acid and olive leaf extract on the quality and shelf-life stability of packaged raw minced beef patties. Meat Sci. 84:613–620. https://doi.org/10.1016/j.meatsci.2009.10.020.
Hunt, M. C., R. A. Mancini, K. A. Hachmeister, D. H. Kropf, M. Merriman, G. DelDuca, and G. Milliken. 2004. Carbon monoxide in modified atmosphere packaging affects color, shelf life, and microorganisms of beef steaks and ground beef. J. Food Sci. 69:FCT45–FCT52. https://doi.org/10.1111/j.1365-2621.2004.tb17854.x.
Hernández, B., C. Sáenz, C. Alberdi, and J. M. Diñeiro. 2015. Comparison between two different methods to obtain the proportions of myoglobin redox forms on fresh meat from reflectance measurements. J. Food Sci. Technol. 52:8212–8219. https://doi.org/10.1007/s13197-015-1917-x.
Holman, B. W. B., D. Collins, A. K. Kilgannon, and D. L. Hopkins. 2018a. The effect of technical replicate (repeats) on Nix Pro Color SensorTM measurement precision for meat: A case-study on aged beef colour stability. Meat Sci. 135:42–45. https://doi.org/10.1016/j.meatsci.2017.09.001.
Holman, B. W. B., J. P. Kerry, and D. L. Hopkins. 2018b. Meat packaging solutions to current industry challenges: A review. Meat Sci. 144:159–168. https://doi.org/10.1016/j.meatsci.2018.04.026.
Hughes, J., F. Clarke, P. Purslow, and R. Warner. 2017. High pH in beef longissimus thoracis reduces muscle fibre transverse shrinkage and light scattering which contributes to the dark colour. Food Res. Int. 101:228–238. https://doi.org/10.1016/j.foodres.2017.09.003.
Hughes, J. M., F. M. Clarke, P. P. Purslow, and R. D. Warner. 2019. Meat color is determined not only by chromatic heme pigments but also by the physical structure and achromatic light scattering properties of the muscle. Compr. Rev. Food Sci. Food Saf. 1–20. https://doi.org/10.1111/1541-4337.12509.
Hughes, J. M., S. K. Oiseth, P. P. Purslow, and R. D. Warner. 2014. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 98:520–532. https://doi.org/10.1016/j.meatsci.2014.05.022.
Ismail, H. A., E. J. Lee, K. Y. Ko, and D. U. Ahn. 2008. Effects of aging time and natural antioxidants on the color, lipid oxidation and volatiles of irradiated ground beef. Meat Sci. 80:582–591. https://doi.org/10.1016/j.meatsci.2008.02.007.
Jeong, J. Y., and J. R. Claus. 2010. Color stability and reversion in carbon monoxide packaged ground beef. Meat Sci. 85:525–530. https://doi.org/10.1016/j.meatsci.2010.02.027.
Jia, N., B. Kong, Q. Liu, X. Diao, and X. Xia. 2012. Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Sci. 91:533–539. https://doi.org/10.1016/j.meatsci.2012.03.010.
John, L., D. Cornforth, C. E. Carpenter, O. Sorheim, B. C. Pettee, and D. R. Whittier. 2005. Color and thiobarbituric acid values of cooked top sirloin steaks packaged in modified atmospheres of 80% oxygen, or 0.4% carbon monoxide, or vacuum. Meat Sci. 69:441–449. https://doi.org/10.1016/j.meatsci.2004.08.013.
Joseph, P., M. N. Nair, and S. P. Suman. 2015. Application of proteomics to characterize and improve color and oxidative stability of muscle foods. Food Res. Int. 76:938–945. https://doi.org/10.1016/j.foodres.2015.05.041.
Joseph, P., S. P. Suman, G. Rentfrow, S. Li, and C. M. Beach. 2012. Proteomics of muscle-specific beef color stability. J. Agr. Food Chem. 60:3196–3203. https://doi.org/10.1021/jf204188v.
Júnior, A., N. Fronza, F. Foralosso, D. Dezen, E. Huber, J. Santos, R. Machado, and M. Quadri. 2015. Biodegradable duo-functional active film: Antioxidant and antimicrobial actions for the conservation of beef. Food Bioprocess Tech. 8:75–87. https://doi.org/10.1007/s11947-014-1376-9.
Ke, Y., R. M. Mitacek, A. Abraham, G. G. Mafi, D. L. VanOverbeke, U. Desilva, and R. Ramanathan. 2017. Effects of muscle-specific oxidative stress on cytochrome c release and oxidation-reduction potential properties. J. Agr. Food Chem. 65:7749–7755. https://doi.org/10.1021/acs.jafc.7b01735.
Khatri, M., V. T. Phung, T. Isaksson, O. Sørheim, E. Slinde, and B. Egelandsdal. 2012. New procedure for improving precision and accuracy of instrumental color measurements of beef. Meat Sci. 91:223–231. https://doi.org/10.1016/j.meatsci.2012.01.012.
Kim, S.-J., A. R. Cho, and J. Han. 2013a. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control. 29:112–120. https://doi.org/10.1016/j.foodcont.2012.05.060.
Kim, Y. H., E. Huff-Lonergan, J. G. Sebranek, and S. M. Lonergan. 2010. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 85:759–767. https://doi.org/10.1016/j.meatsci.2010.04.001.
Kim, Y. H., M. C. Hunt, R. A. Mancini, M. Seyfert, T. M. Loughin, D. H. Kropf, and J. S. Smith. 2006. Mechanism for lactate-color stabilization in injection-enhanced beef. J. Agr. Food Chem. 54:7856–7862. https://doi.org/10.1021/jf061225h.
Kim, Y. H., J. T. Keeton, H. S. Yang, S. B. Smith, J. E. Sawyer, and J. W. Savell. 2009. Color stability and biochemical characteristics of bovine muscles when enhanced with L- or D-potassium lactate in high-oxygen modified atmospheres. Meat Sci. 82:234–240. https://doi.org/10.1016/j.meatsci.2009.01.016.
Kim, Y. H. B., B. Meyers, H. W. Kim, A. M. Liceaga, and R. P. Lemenager. 2017. Effects of stepwise dry/wet-aging and freezing on meat quality of beef loins. Meat Sci. https://doi.org/10.1016/j.meatsci.2016.09.002.
Kim, S.-J., S. C. Min, H.-J. Shin, Y.-J. Lee, A. R. Cho, S. Y. Kim, and J. Han. 2013b. Evaluation of the antioxidant activities and nutritional properties of ten edible plant extracts and their application to fresh ground beef. Meat Sci. 93:715–722. https://doi.org/10.1016/j.meatsci.2012.11.029.
King, D. A., S. D. Shackelford, L. A. Kuehn, C. M. Kemp, A. B. Rodriguez, R. M. Thallman, and T. L. Wheeler. 2010. Contribution of genetic influences to animal-to-animal variation in myoglobin content and beef lean color stability. J. Anim. Sci. 88:1160–1167. https://doi.org/10.2527/jas.2009-2544.
King, D. A., S. D. Shackelford, A. B. Rodriguez, and T. L. Wheeler. 2011. Effect of time of measurement on the relationship between metmyoglobin reducing activity and oxygen consumption to instrumental measures of beef longissimus color stability. Meat Sci. 87:26–32. https://doi.org/10.1016/j.meatsci.2010.08.013.
Knock, R. C., M. Seyfert, M. C. Hunt, M. E. Dikeman, R. A. Mancini, J. A. Unruh, J. J. Higgins, and R. A. Monderen. 2006. Effects of potassium lactate, sodium chloride, sodium tripolyphosphate, and sodium acetate on colour, colour stability, and oxidative properties of injection-enhanced beef rib steaks. Meat Sci. 74:312–318. https://doi.org/10.1016/j.meatsci.2006.03.029.
Krzywicki, K. 1979. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 3:1–10. https://doi.org/10.1016/0309-1740(79)90019-6.
Le Bihan-Duval, E., J. Nadaf, C. Berri, F. Pitel, B. Graulet, E. Godet, S. Y. Leroux, O. Demeure, S. Lagarrigue, C. Duby, L. A. Cogburn, C. M. Beaumont, and M. J. Duclos. 2011. Detection of a cis eQTL controlling BMCO1 gene expression leads to the identification of a QTG for chicken breast meat color. P. H. Reitsma, editor. PLoS One. 6:e14825. https://doi.org/10.1371/journal.pone.0014825.
Ledward, D. A. 1970. Metmyoglobin formation in beef stored in carbon dioxide enriched and oxygen depleted atmospheres. J. Food Sci. 35:33–37. https://doi.org/10.1111/j.1365-2621.1970.tb12362.x.
Ledward, D. A. 1972. Metmyoglobin reduction and formation in beef during aerobic storage at l°C. J. Food Sci. 37:634–635. https://doi.org/10.1111/j.1365-2621.1972.tb02713.x.
Li, X., M. Ekerljung, K. Lundström, and A. Lundén. 2013. Association of polymorphisms at DGAT1, leptin, SCD1, CAPN1 and CAST genes with color, marbling and water holding capacity in meat from beef cattle populations in Sweden. Meat Sci. 94:153–158. https://doi.org/10.1016/j.meatsci.2013.01.010.
Li, Z., M. Li, X. Li, J. Xin, Y. Wang, Q. W. Shen, and D. Zhang. 2018. Quantitative phosphoproteomic analysis among muscles of different color stability using tandem mass tag labeling. Food Chem. 249:8–15. https://doi.org/10.1016/j.foodchem.2017.12.047.
Liu, C., Y. Zhang, X. Yang, R. Liang, Y. Mao, X. Hou, X. Lu, and X. Luo. 2014. Potential mechanisms of carbon monoxide and high oxygen packaging in maintaining color stability of different bovine muscles. Meat Sci. 97:189–196. https://doi.org/10.1016/j.meatsci.2014.01.027.
Ma, D., Y. H. B. Kim, B. Cooper, J. H. Oh, H. Chun, J. H. Choe, J. P. Schoonmaker, K. Ajuwon, and B. Min. 2017. Metabolomics profiling to determine the effect of postmortem aging on color and lipid oxidative stabilities of different bovine muscles. J. Agr. Food Chem. 65:6708–6716. https://doi.org/10.1021/acs.jafc.7b02175.
Magalhães, A. F. B., F. S. Schenkel, D. A. Garcia, D. G. M. Gordo, R. L. Tonussi, R. Espigolan, R. M. de O. Silva, C. U. Braz, G. A. Fernandes Júnior, F. Baldi, R. Carvalheiro, A. A. Boligon, H. N. de Oliveira, L. A. L. Chardulo, and L. G. de Albuquerque. 2019. Genomic selection for meat quality traits in Nelore cattle. Meat Sci. 148:32–37. https://doi.org/10.1016/j.meatsci.2018.09.010.
Mahmood, S., B. C. Roy, I. L. Larsen, J. L. Aalhus, W. T. Dixon, and H. L. Bruce. 2017. Understanding the quality of typical and atypical dark cutting beef from heifers and steers. Meat Sci. 133:75–85. https://doi.org/10.1016/j.meatsci.2017.06.010.
Mancini, R. A., and M. C. Hunt. 2005. Current research in meat color. Meat Sci. 71:100–121. https://doi.org/10.1016/j.meatsci.2005.03.003.
Mancini, R. A., and R. Ramanathan. 2014. Effects of postmortem storage time on color and mitochondria in beef. Meat Sci. 98:65–70. https://doi.org/10.1016/j.meatsci.2014.04.007.
Mancini, R. A., R. Ramanathan, S. P. Suman, G. Dady, and P. Joseph. 2011. Effects of succinate on ground beef color and premature browning. Meat Sci. 89:189–194. https://doi.org/https://doi.org/10.1016/j.meatsci.2011.04.016.
Mancini, R. A., R. Ramanathan, S. P. Suman, M. K. R. Konda, P. Joseph, G. A. Dady, B. M Naveena, and I. López-López. 2010. Effects of lactate and modified atmospheric packaging on premature browning in cooked ground beef patties. Meat Sci. 85:339–346. https://doi.org/10.1016/j.meatsci.2010.02.001.
Mancini, R. A., S. P. Suman, M. K. R. Konda, and R. Ramanathan. 2009. Effect of carbon monoxide packaging and lactate enhancement on the color stability of beef steaks stored at 1 °C for 9 days. Meat Sci. 81:71–76. https://doi.org/10.1016/j.meatsci.2008.06.021.
Mansour, E. H., and A. H. Khalil. 2000. Evaluation of antioxidant activity of some plant extracts and their application to ground beef patties. Food Chem. 69:135–141. https://doi.org/10.1016/S0308-8146(99)00234-4.
Marino, R., M. Albenzio, A. della Malva, M. Caroprese, A. Santillo, and A. Sevi. 2014. Changes in meat quality traits and sarcoplasmic proteins during aging in three different cattle breeds. Meat Sci. 98:178–186. https://doi.org/10.1016/j.meatsci.2014.05.024.
McKeith, R. O., D. A. King, A. L. Grayson, S. D. Shackelford, K. B. Gehring, J. W. Savell, and T. L. Wheeler. 2016. Mitochondrial abundance and efficiency contribute to lean color of dark cutting beef. Meat Sci. 116:165–173. https://doi.org/10.1016/j.meatsci.2016.01.016.
McKenna, D. R., P. D. Mies, B. E. Baird, K. D. Pfeiffer, J. W. Ellebracht, and J. W. Savell. 2005. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 70:665–682. https://doi.org/10.1016/j.meatsci.2005.02.016.
McMillin, K. W. 2008. Where is MAP going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 80:43–65. https://doi.org/10.1016/j.meatsci.2008.05.028.
McMillin, K. W. 2017. Advancements in meat packaging. Meat Sci. 132:153–162. https://doi.org/10.1016/j.meatsci.2017.04.015.
Mitacek, R. M., A. R. English, G. G. Mafi, D. L. VanOverbeke, and R. Ramanathan. 2018. Modified atmosphere packaging improves surface color of dark-cutting beef. Meat Muscle Biology. 2:57. https://doi.org/10.22175/mmb2017.04.0023.
Mitacek, R. M., Y. Ke, J. E. Prenni, R. Jadeja, D. L. VanOverbeke, G. G. Mafi, and R. Ramanathan. 2019. Mitochondrial degeneration, depletion of NADH, and oxidative stress decrease color stability of wet-aged beef longissimus steaks. J. Food Sci. 84:38–50. https://doi.org/10.1111/1750-3841.14396.
Mohan, A., M. C. Hunt, T. J. Barstow, T. A. Houser, and D. M. Hueber. 2010a. Near-infrared oximetry of three post-rigor skeletal muscles for following myoglobin redox forms. Food Chem. 123:456–464. https://doi.org/10.1016/j.foodchem.2010.04.068.
Mohan, A., M. C. Hunt, T. J. Barstow, T. A. Houser, and S. Muthukrishnan. 2010b. Effects of malate, lactate, and pyruvate on myoglobin redox stability in homogenates of three bovine muscles. Meat Sci. 86:304–310. https://doi.org/10.1016/j.meatsci.2010.04.030.
Mohan, A., S. Muthukrishnan, M. C. Hunt, T. J. Barstow, and T. A. Houser. 2010c. Kinetics of myoglobin redox form stabilization by malate dehydrogenase. J. Agr. Food Chem. 58:6994–7000. https://doi.org/10.1021/jf100639n.
Muroya, S., M. Oe, I. Nakajima, K. Ojima, and K. Chikuni. 2014. CE-TOF MS-based metabolomic profiling revealed characteristic metabolic pathways in postmortem porcine fast and slow type muscles. Meat Sci. 98:726–735. https://doi.org/10.1016/j.meatsci.2014.07.018.
Nair, M. N., B. R. C. Costa-Lima, M. Wes Schilling, and S. P. Suman. 2017a. Proteomics of color in fresh muscle foods. In: Proteomics in Food Science: From Farm to Fork. Academic Press, Cambridge, MA. p. 163–175.
Nair, M. N., S. Li, C. Beach, G. Rentfrow, and S. P. Suman. 2018. Intramuscular variations in color and sarcoplasmic proteome of beef during postmortem aging. Meat Muscle Biology. 2:92–101. https://doi.org/10.22175/mmb2017.11.0055.
Nair, M. N., R. Ramanathan, G. Rentfrow, and S. P. Suman. 2017b. Intramuscular variation in mitochondrial functionality of beef semimembranosus. South African J. Anim. Sci. 47:635–639. https://doi.org/10.4314/sajas.v47i5.6.
Nair, M. N., S. P. Suman, M. K. Chatli, S. Li, P. Joseph, C. M. Beach, and G. Rentfrow. 2016. Proteome basis for intramuscular variation in color stability of beef semimembranosus. Meat Sci. 113:9–16. https://doi.org/10.1016/j.meatsci.2015.11.003.
Nassu, R. T., B. Uttaro, J. L. Aalhus, S. Zawadski, M. Juárez, and M. E. R. Dugan. 2012. Type of packaging affects the colour stability of vitamin E enriched beef. Food Chem. 135:1868–1872. https://doi.org/10.1016/j.foodchem.2012.06.055.
Neethling, N. E., S. P. Suman, G. O. Sigge, L. C. Hoffman, and M. C. Hunt. 2017. Exogenous and endogenous factors influencing color of fresh meat from ungulates. Meat Muscle Biology. 1:253–275. https://doi.org/10.22175/mmb2017.06.0032.
Nerín, C., L. Tovar, D. Djenane, J. Camo, J. Salafranca, J. A. Beltrán, and P. Roncalés. 2006. Stabilization of beef meat by a new active packaging containing natural antioxidants. J. Agr. Food Chem. 54:7840–7846. https://doi.org/10.1021/jf060775c.
Nguyen, T., S. Kim, and J. G. Kim. 2019. Diffuse reflectance spectroscopy to quantify the met-myoglobin proportion and meat oxygenation inside of pork and beef. Food Chem. 275:369–376. https://doi.org/10.1016/j.foodchem.2018.09.121.
Nisa, I., B. Ashwar, A. Shah, A. Gani, A. Gani, and F. Masoodi. 2015. Development of potato starch based active packaging films loaded with antioxidants and its effect on shelf life of beef. J. Food Sci. Tech. 52:7245–7253. https://doi.org/10.1007/s13197-015-1859-3.
Nonneman, D. J., S. D. Shackelford, D. A. King, T. L. Wheeler, R. T. Wiedmann, W. M. Snelling, and G. A. Rohrer. 2013. Genome-wide association of meat quality traits and tenderness in swine. J. Anim. Sci. 91:4043–4050. https://doi.org/10.2527/jas.2013-6255.
O’Grady, M. N., F. J. Monahan, R. M. Burke, and P. Allen. 2000. The effect of oxygen level and exogenous α-tocopherol on the oxidative stability of minced beef in modified atmosphere packs. Meat Sci. 55:39–45. https://doi.org/10.1016/S0309-1740(99)00123-0.
Park, H. Y., S. J. Kim, K. M. Kim, Y. S. You, S. Y. Kim, and J. Han. 2012. Development of antioxidant packaging material by applying corn-zein to LLDPE film in combination with phenolic compounds. J. Food Sci. 77:E273–E279. https://doi.org/10.1111/j.1750-3841.2012.02906.x.
Ponnampalam, E. N., D. L. Hopkins, H. Bruce, D. Li, G. Baldi, and A. E. din Bekhit. 2017. Causes and contributing factors to “dark cutting” meat: Current trends and future directions: A review. Compr. Rev. Food Sci. Food Saf. 16:400–430. https://doi.org/10.1111/1541-4337.12258.
Prill, L. L., L. N. Drey, B. A. Olson, E. A. Rice, J. M. Gonzalez, J. L. Vipham, M. D. Chao, P. D. Bass, M. J. Colle, and T. G. O. 2019. Visual degree of doneness impacts beef palatability for consumers with different degree of doneness preferences. Meat Muscle Biology. 3:411–423. https://doi.org/10.22175/mmb2019.07.0024.
Prommachart, R., T. S. Belem, S. Uriyapongson, P. Rayas-Duarte, J. Uriyapongson, R. Ramanathan. 2020. The effect of black rice water extract on surface color, lipid oxidation, microbial growth, and antioxidant activity of beef patties during chilled storage. Meat Sci. 164:108091. https://doi.org/10.1016/j.meatsci.2020.108091.
Purslow, P. P., R. D. Warner, F. M. Clarke, and J. M. Hughes. 2020. Variations in meat colour due to factors other than myoglobin chemistry; a synthesis of recent findings (invited review). Meat Sci. 159:107941. https://doi.org/10.1016/j.meatsci.2019.107941.
Raines, C. R., M. C. Hunt, and J. A. Unruh. 2010. Contributions of muscles of various color stabilities to the overall color stability of ground beef. J. Food Sci. 75:C85–C89. https://doi.org/10.1111/j.1750-3841.2009.01430.x.
Ramanathan, R., M. C. Hunt, A. R. English, G. G. Mafi, and D. L. VanOverbeke. 2019a. Effects of aging, modified atmospheric packaging, and display time on metmyoglobin reducing activity and oxygen consumption of high-pH beef. Meat Muscle Biology. 3:276. https://doi.org/10.22175/mmb2019.05.0017.
Ramanathan, R., and R. A. Mancini. 2018. Role of mitochondria in beef color: A review. Meat Muscle Biology. 2:309. https://doi.org/10.22175/mmb2018.05.0013.
Ramanathan, R., R. A. Mancini, and G. A. Dady. 2011. Effects of pyruvate, succinate, and lactate enhancement on beef longissimus raw color. Meat Sci. 88:424–428. https://doi.org/10.1016/j.meatsci.2011.01.021.
Ramanathan, R., R. A. Mancini, P. Joseph, and S. P. Suman. 2013. Bovine mitochondrial oxygen consumption effects on oxymyoglobin in the presence of lactate as a substrate for respiration. Meat Sci. 93:893–897. https://doi.org/10.1016/j.meatsci.2012.12.005.
Ramanathan, R., R. A. Mancini, and M. R. Konda. 2009. Effects of lactate on beef heart mitochondrial oxygen consumption and muscle darkening. J. Agr. Food Chem. 57:1550–1555. https://doi.org/10.1021/jf802933p.
Ramanathan, R., R. A. Mancini, B. M. Naveena, and M. K. R. Konda. 2010. Effects of lactate-enhancement on surface reflectance and absorbance properties of beef longissimus steaks. Meat Sci. 84:219–226. https://doi.org/10.1016/j.meatsci.2009.08.027.
Ramanathan, R., R. A. Mancini, S. P. Suman, and C. M. Beach. 2014. Covalent binding of 4-hydroxy-2-nonenal to lactate dehydrogenase decreases nadh formation and metmyoglobin reducing activity. J. Agr. Food Chem. 62:2112–2117. https://doi.org/10.1021/jf404900y.
Ramanathan, R., R. A. Mancini, C. B. Van Buiten, S. P. Suman, and C. M. Beach. 2012. Effects of pyruvate on lipid oxidation and ground beef color. J. Food Sci. 77:C886–C892. https://doi.org/10.1111/j.1750-3841.2012.02814.x
Ramanathan, R., R. M. Mitacek, S. D. Billups, R. Jadeja, M. M. Pfeiffer, G. G. Mafi, and D. L. VanOverbeke. 2018. Novel nitrite-embedded packaging improves surface redness of dark-cutting longissimus steaks. Transl. Anim. Sci. 2:135–143. https://doi.org/10.1093/tas/txy006.
Ramanathan, R., M. N. Nair, M. C. Hunt, and S. P. Suman. 2019b. Mitochondrial functionality and beef colour: A review of recent research. S. Afr. J. Anim. Sci. 49:9. https://doi.org/10.4314/sajas.v49i1.2.
Ramanathan, R., S. P. Suman, and C. Faustman. 2020. Biomolecular interactions governing fresh meat color in post-mortem skeletal muscle: A review. J. Agr. Food Chem. https://doi.org/10.1021/acs.jafc.9b08098.
Reiley, L. 2019. Impossible burger: Here’s what’s really in it. Washington Post. https://www.washingtonpost.com/business/2019/10/23/an-impossible-burger-dissected/. (Accessed 23 October 2019).
Resconi, V. C., A. Escudero, J. A. Beltrán, J. L. Olleta, C. Sañudo, and M. D. Mar Campo. 2012. Color, lipid oxidation, sensory quality, and aroma compounds of beef steaks displayed under different levels of oxygen in a modified atmosphere package. J. Food Sci. 77:S10–S18. https://doi.org/10.1111/j.1750-3841.2011.02506.x
Sammel, L. M., M. C. Hunt, D. H. Kropf, K. A. Hachmeister, and D. E. Johnson. 2002. Comparison of assays for metmyoglobin reducing ability in beef inside and outside semimembranosus muscle. J. Food Sci. 67:978–984. https://doi.org/10.1111/j.1365-2621.2002.tb09439.x.
Sanchez, M.-P., T. Tribout, N. Iannuccelli, M. Bouffaud, B. Servin, A. Tenghe, P. Dehais, N. Muller, M. Del Schneider, M.-J. Mercat, C. Rogel-Gaillard, D. Milan, J.-P. Bidanel, and H. Gilbert. 2014. A genome-wide association study of production traits in a commercial population of Large White pigs: evidence of haplotypes affecting meat quality. Genet. Sel. Evol. 46:12. https://doi.org/10.1186/1297-9686-46-12.
Sánchez-Escalante, A., D. Djenane, G. Torrescano, J. A. Beltrán, and P. Roncalés. 2001. The effects of ascorbic acid, taurine, carnosine and rosemary powder on colour and lipid stability of beef patties packaged in modified atmosphere. Meat Sci. 58:421–429. https://doi.org/10.1016/S0309-1740(01)00045-6.
Sánchez-Escalante, A., D. Djenane, G. Torrescano, J. A. Beltrán, and P. Roncales. 2003. Antioxidant action of borage, rosemary, oregano, and ascorbic acid in beef patties packaged in modified atmosphere. J. Food Sci. 68:339–344. https://doi.org/10.1111/j.1365-2621.2003.tb14162.x.
Sánchez-Escalante, A., G. Torrescano, D. Djenane, J. A. Beltrán, B. Giménez, and P. Roncalés. 2011. Effect of antioxidants and lighting conditions on color and lipid stability of beef patties packaged in high-oxygen modified atmosphere. J. Food Sci. 9:49–57. https://doi.org/10.1080/19476330903572945.
Sawyer, J. T., J. K. Apple, Z. B. Johnson, R. T. Baublits, and J. W. S. Yancey. 2009. Fresh and cooked color of dark-cutting beef can be altered by post-rigor enhancement with lactic acid. Meat Sci. 83:263–270. https://doi.org/10.1016/j.meatsci.2009.05.008.
Sawyer, J. T., J. W. S. Yancey, M. D. Wharton, J. K. Apple, and J.-F. Meullenet. 2011. Lactic acid enhancement can improve the fresh and cooked color of dark-cutting beef. J. Anim. Sci. 89:4207–4220. https://doi.org/10.2527/jas.2011-4147.
Sayd, T., M. Morzel, C. Chambon, M. Franck, P. Figwer, C. Larzul, P. Le Roy, G. Monin, P. Chérel, and E. Laville. 2006. Proteome analysis of the sarcoplasmic fraction of pig Semimembranosus muscle: Implications on meat color development. J. Agr. Food Chem. 54:2732–2737. https://doi.org/10.1021/jf052569v.
Seyfert, M., R. A. Mancini, M. C. Hunt, J. Tang, and C. Faustman. 2007. Influence of carbon monoxide in package atmospheres containing oxygen on colour, reducing activity, and oxygen consumption of five bovine muscles. Meat Sci. 75:432–442. https://doi.org/10.1016/j.meatsci.2006.08.007.
Seyfert, M., R. A. Mancini, M. C. Hunt, J. Tang, C. Faustman, and M. Garcia. 2006. Color stability, reducing activity, and cytochrome c oxidase activity of five bovine muscles. J. Agr. Food Chem. 54:8919–8925. https://doi.org/10.1021/jf061657s.
Sharma, V., N. Lee, G. Nagaraj, R. Singh, and A. Mohan. 2014. Effects of injection enhancement with sodium tripolyphosphate, carrageenan, and sea salt on beef retail display properties and color stability. Meat Sci. 96:476–477. https://doi.org/10.1016/j.meatsci.2013.07.117.
Sirocchi, V., F. Devlieghere, N. Peelman, G. Sagratini, F. Maggi, S. Vittori, and P. Ragaert. 2017. Effect of Rosmarinus officinalis L. essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chem. 221:1069–1076. https://doi.org/10.1016/j.foodchem.2016.11.054.
Stackhouse, R., J. Apple, J. Yancey, C. Keys, T. Johnson, and L. Mehall. 2016. Postrigor citric acid enhancement can alter cooked color but not fresh color of dark-cutting beef. J. Anim. Sci. 94:1738–1754. https://doi.org/10.2527/jas.2015-0181.
Subbaraj, A. K., Y. H. B. Kim, K. Fraser, and M. M. Farouk. 2016. A hydrophilic interaction liquid chromatography-mass spectrometry (HILIC-MS) based metabolomics study on colour stability of ovine meat. Meat Sci. 117:163–72. https://doi.org/10.1016/j.meatsci.2016.02.028.
Suman, S. P., C. Faustman, S. L. Stamer, and D. C. Liebler. 2007. Proteomics of lipid oxidation-induced oxidation of porcine and bovine oxymyoglobins. Proteomics. 7:628–640. https://doi.org/10.1002/pmic.200600313.
Suman, S. P., M. C. Hunt, M. N. Nair, and G. Rentfrow. 2014. Improving beef color stability: Practical strategies and underlying mechanisms. Meat Sci. 98:490–504. https://doi.org/10.1016/j.meatsci.2014.06.032.
Suman, S. P., and P. Joseph. 2013. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 4:79–99. https://doi.org/10.1146/annurev-food-030212-182623.
Suman, S. P., R. A. Mancini, P. Joseph, R. Ramanathan, M. K. R. Konda, G. Dady, B. M. Naveena, and I. López-López. 2010a. Color-stabilizing effect of lactate on ground beef is packaging-dependent. Meat Sci. 84:329–333. https://doi.org/10.1016/j.meatsci.2009.08.051.
Suman, S. P., R. A. Mancini, P. Joseph, R. Ramanathan, M. K. R. Konda, G. Dady, and S. Yin. 2010b. Packaging-specific influence of chitosan on color stability and lipid oxidation in refrigerated ground beef. Meat Sci. 86:994–998. https://doi.org/10.1016/j.meatsci.2010.08.006.
Suman, S. P., M. N. Nair, P. Joseph, and M. C. Hunt. 2016. Factors influencing internal color of cooked meats. Meat Sci. 120:133–144. https://doi.org/10.1016/j.meatsci.2016.04.006.
Swatland, H. J. 1989. A review of meat spectrophotometry (300 to 800 nm). Can. Inst. Food Sci. Technol. J. 22:390–402. https://doi.org/10.1016/s0315-5463(89)70435-1.
Swatland, H. J. 2003. Fibre-optic spectrophotometry of beef relative to sarcomere length (short communication). Archiv fur Tierzucht. 46:31–34. https://doi.org/10.5194/aab-46-31-2003.
Swatland, H. J. 2008. How pH causes paleness or darkness in chicken breast meat. Meat Sci. 80:396–400. https://doi.org/10.1016/j.meatsci.2008.01.002.
Tang, J., C. Faustman, T. A. Hoagland, R. A. Mancini, M. Seyfert, and M. C. Hunt. 2005. Postmortem oxygen consumption by mitochondria and its effects on myoglobin form and stability. J. Agr. Food Chem. 53:1223–1230. https://doi.org/10.1021/jf048646o.
Tapp, W. N., C. T. Christjohn, D. A. Griffing, and C. L. Bratcher. 2017. Evaluation of meat quality on high pH strip loins injected with buffered acetic acid. Meat Muscle Biology. 1:218–226. https://doi.org/10.22175/mmb2017.04.0020.
Tapp, W. N., J. W. S. Yancey, J. K. Apple, M. E. Dikeman, and R. G. Godbee. 2012. Noni puree (Morinda citrifolia) mixed in beef patties enhanced color stability. Meat Sci. 91:131–136. https://doi.org/10.1016/j.meatsci.2012.01.005.
Tomasevic, I., V. Tomovic, P. Ikonic, J. M. Lorenzo Rodriguez, F. J. Barba, I. Djekic, I. Nastasijevic, S. Stajic, and D. Zivkovic. 2019a. Evaluation of poultry meat colour using computer vision system and colourimeter: Is there a difference? Br. Food J. 121:1078–1087. https://doi.org/10.1108/BFJ-06-2018-0376.
Tomasevic, I., V. Tomovic, B. Milovanovic, J. Lorenzo, V. Đorđević, N. Karabasil, and I. Djekic. 2019b. Comparison of a computer vision system vs. traditional colorimeter for color evaluation of meat products with various physical properties. Meat Sci. 148:5–12. https://doi.org/10.1016/j.meatsci.2018.09.015.
Viana, E. S., L. A. M. Gomide, and M. C. D. Vanetti. 2005. Effect of modified atmospheres on microbiological, color and sensory properties of refrigerated pork. Meat Sci. 71:696–705. https://doi.org/10.1016/j.meatsci.2005.05.013.
Van Rooyen, L. A., P. Allen, and D. I. O’Connor. 2017. The application of carbon monoxide in meat packaging needs to be re-evaluated within the EU: An overview. Meat Sci. 132:179–188. https://doi.org/10.1016/j.meatsci.2017.03.016.
Warner, R. D., G. Kearney, D. L. Hopkins, and R. H. Jacob. 2017. Retail colour stability of lamb meat is influenced by breed type, muscle, packaging and iron concentration. Meat Sci. 129:28–37. https://doi.org/10.1016/j.meatsci.2017.01.008.
Wheeler, T. L., M. Koohmaraie, and S. D. Shackelford. 1996. Effect of vitamin C concentration and co-injection with calcium chloride on beef retail display color. J. Anim. Sci. 74:1846–1853. https://doi.org/10.2527/1996.7481846x.
Wills, K. M., R. M. Mitacek, G. G. Mafi, D. L. VanOverbeke, D. Jaroni, R. Jadeja, and R. Ramanathan. 2017. Improving the lean muscle color of dark-cutting beef by aging, antioxidant-enhancement, and modified atmospheric packaging. J. Anim. Sci. 95:5378–5387. https://doi.org/10.2527/jas2017.1967.
Wu, W., X. G. Gao, Y. Dai, Y. Fu, X. M. Li, and R. T. Dai. 2015. Post-mortem changes in sarcoplasmic proteome and its relationship to meat color traits in M. semitendinosus of Chinese Luxi yellow cattle. Food Res. Int. 72:98–105. https://doi.org/10.1016/j.foodres.2015.03.030.
Wu, W., Q. Q. Yu, Y. Fu, X. J. Tian, F. Jia, X. M. Li, and R. T. Dai. 2016. Towards muscle-specific meat color stability of Chinese Luxi yellow cattle: A proteomic insight into post-mortem storage. J. Proteomics. 147:108–118. https://doi.org/10.1016/j.jprot.2015.10.027.
Yang, X., D. R. Woerner, J. D. Hasty, K. R. McCullough, I. Geornaras, J. N. Sofos, and K. E. Belk. 2016a. An evaluation of the effectiveness of FreshCase technology to extend the storage life of whole muscle beef and ground beef. J. Anim. Sci. 94:4911–4920. https://doi.org/10.2527/jas.2016-0508.
Yang, X., D. R. Woerner, K. R. McCullough, J. D. Hasty, I. Geornaras, G. C. Smith, J. N. Sofos, and K. E. Belk. 2016b. An evaluation of the effectiveness of FreshCase technology to extend the storage life of whole-muscle pork and ground pork sausage. J. Anim. Sci. 94:4921–4929. https://doi.org/10.2527/jas.2016-0509.
Yang, X., S. Wu, D. L. Hopkins, R. Liang, L. Zhu, Y. Zhang, and X. Luo. 2018. Proteomic analysis to investigate color changes of chilled beef longissimus steaks held under carbon monoxide and high oxygen packaging. Meat Sci. 142:23–31. https://doi.org/10.1016/j.meatsci.2018.04.001.
Yıldız-Turp, G., and M. Serdaroglu. 2010. Effects of using plum puree on some properties of low fat beef patties. Meat Sci. 86:896–900. https://doi.org/10.1016/j.meatsci.2010.07.009.
Yu, Q., W. Wu, X. Tian, M. Hou, R. Dai, and X. Li. 2017a. Unraveling proteome changes of Holstein beef M. semitendinosus and its relationship to meat discoloration during post-mortem storage analyzed by label-free mass spectrometry. J. Proteomics. 154:85–93. https://doi.org/10.1016/j.jprot.2016.12.012.
Yu, Q., W. Wu, X. Tian, F. Jia, L. Xu, R. Dai, and X. Li. 2017b. Comparative proteomics to reveal muscle-specific beef color stability of Holstein cattle during post-mortem storage. Food Chem. 229:769–778. https://doi.org/10.1016/j.foodchem.2017.03.004.
Zhai, C., K. Peckham, K. E. Belk, R. Ramanathan, and M. N. Nair. 2019. Carbon chain length of lipid oxidation products influence lactate dehydrogenase and NADH-dependent metmyoglobin reductase activity. J. Agr. Food Chem. 67:13327–13332. https://doi.org/10.1021/acs.jafc.9b05634.
Zhang, Y., L. Qin, Y. Mao, D. L. Hopkins, G. Han, L. Zhu, and X. Luo. 2018. Carbon monoxide packaging shows the same color improvement for dark cutting beef as high oxygen packaging. Meat Sci. 137:153–159. https://doi.org/10.1016/j.meatsci.2017.11.016.
Zinoviadou, K. G., K. P. Koutsoumanis, and C. G. Biliaderis. 2009. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 82:338–345. https://doi.org/10.1016/j.meatsci.2009.02.004.
Supplementary data
Calculation to determine the ratio of myoglobin and carbon monoxide concentration in modified atmospheric packaging
Average loin eye area = 14 inch2 = 90 cm2
Assuming CO can penetrate 2 mm from surface
Total volume of meat = 90 cm2 × 0. 2 cm = 18 cm3 = 18 mL = 0.018 L
Average myoglobin concentration = 2.5 mg/g
Therefore, concentration in 18 mL = 0.15 × 10-3 M/L X 18 × 10-3 L = 0.027 mM
Volume of MAP tray = 1065 mL (determined by water displacement method)
Volume of 2.5 thick steak = 350 mL
Volume of gas occupied = 1065-350 = 715 cm3 = 0.715 liter
Number of moles in headspace was calculated according to Raines et al., 2010.
| CO-MAP (0.4% CO) | PVC (20% oxygen) | High-oxygen (80% oxygen) |
|---|---|---|
| Number of moles in head space calculated as PV = nRT where P is the pressure in Pascal, V is volume in liters, n = number of moles of gas, R is ideal gas constant and T is temperature in Kelvin |
Number of moles in head space calculated as PV = nRT where P is the pressure in Pascal, V is volume in liters, n = number of moles of gas, R is ideal gas constant and T is temperature in Kelvin |
Number of moles in head space calculated as PV = nRT where P is the pressure in Pascal, V is volume in liters, n = number of moles of gas, R is ideal gas constant and T is temperature in Kelvin |
| Pressure of CO gas in the package = 60 psi = 413.6 kPas 413.6 × 0.715 = n × 8,314.4 × 277.15 n= 1.26 × 10-6 moles CO level in the gas mixture = 0.4% Therefore, number of moles = 0.05 mM |
Pressure of oxygen in atmosphere = 101.325 kPa 101.325 × 0.715 = n × 8,314.4 × 277.15 n= 3.14 × 10-5 moles Oxygen level in atmosphere = 20% Therefore, number of moles = 0.628 mM |
Pressure of CO gas in the package = 60 psi = 413.6 kPas 413.6 × 0.715 = n × 8,314.4 × 277.15 n= 1.26 × 10-6 moles Oxygen level in the gas mixture = 80% Therefore, number of moles = 10 mM |
| Mb: CO = 0.027: 0.05 = 1:1.85 | Mb: O2 = 0.027: 0.628 = 1:23.2 | Mb: O2 = 0.027: 10 = 1:370 |
| For each mole of myoglobin there will be approximately 1.85 moles of CO. Not enough CO molecules to saturate myoglobin | For each mole of myoglobin there will be approximately 23 moles of oxygen to bind | For each mole of myoglobin there will be approximately 370 moles of oxygen |